Compositions and Methods for Treating and Preventing Pancreatitis, Renal Injury and Cancer
a technology of renal injury and compositions, applied in the field of compositions and methods for treating and preventing pancreatitis, renal injury and cancer, can solve the problems of increasing morbidity and mortality, worsening severity, and increasing the activity of ckd, so as to reduce the the level of activity of a renalase receptor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
experimental examples
[0227]The invention is further described in detail by reference to the following experimental examples. These examples are provided for purposes of illustration only, and are not intended to be limiting unless otherwise specified. Thus, the invention should in no way be construed as being limited to the following examples, but rather, should be construed to encompass any and all variations which become evident as a result of the teaching provided herein.
[0228]Without further description, it is believed that one of ordinary skill in the art can, using the preceding description and the following illustrative examples, make and utilize the present invention and practice the claimed methods. The following working examples therefore, specifically point out the preferred embodiments of the present invention, and are not to be construed as limiting in any way the remainder of the disclosure.
example 1
Identification of a Receptor for Extracellular Renalase
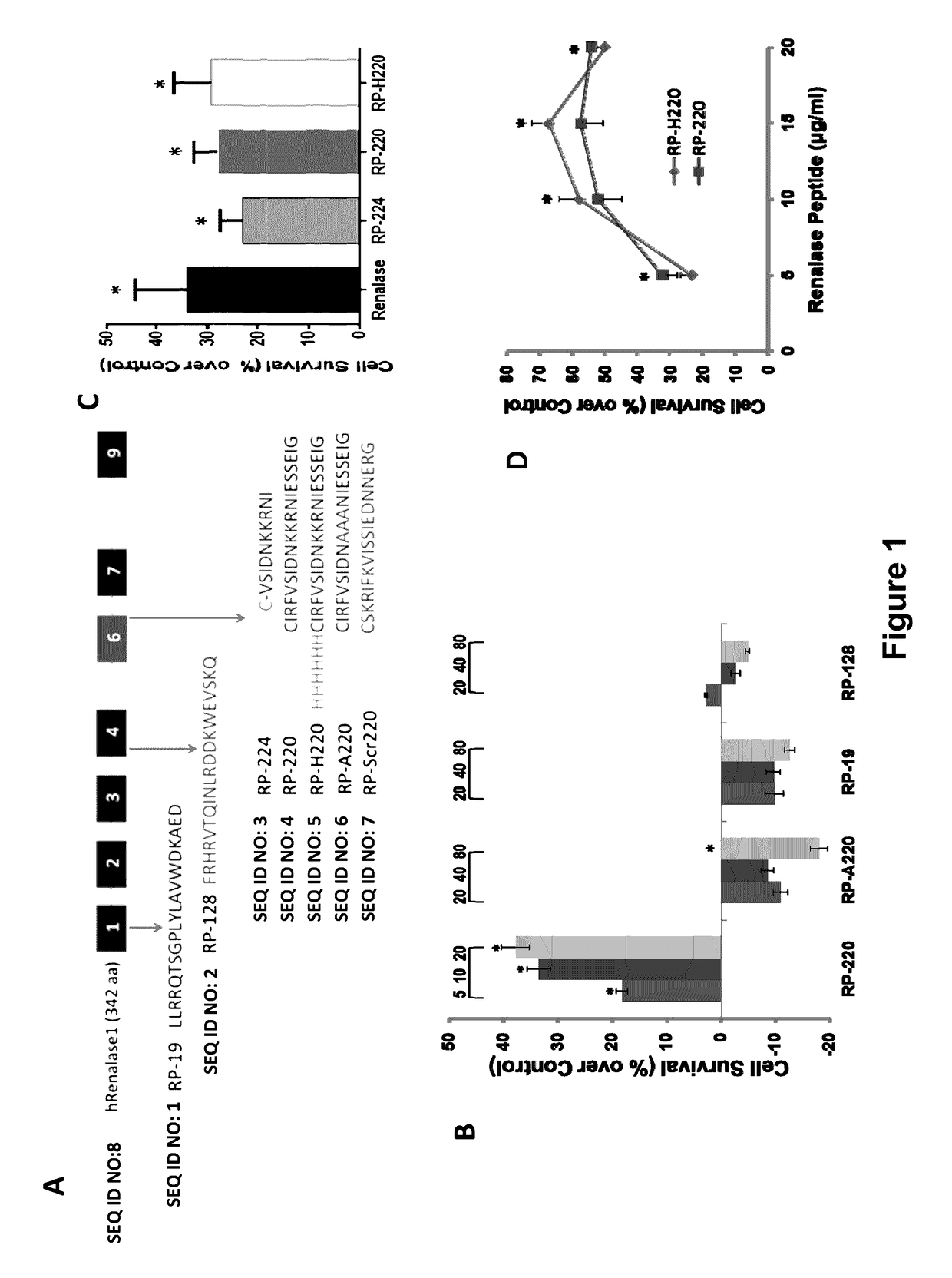
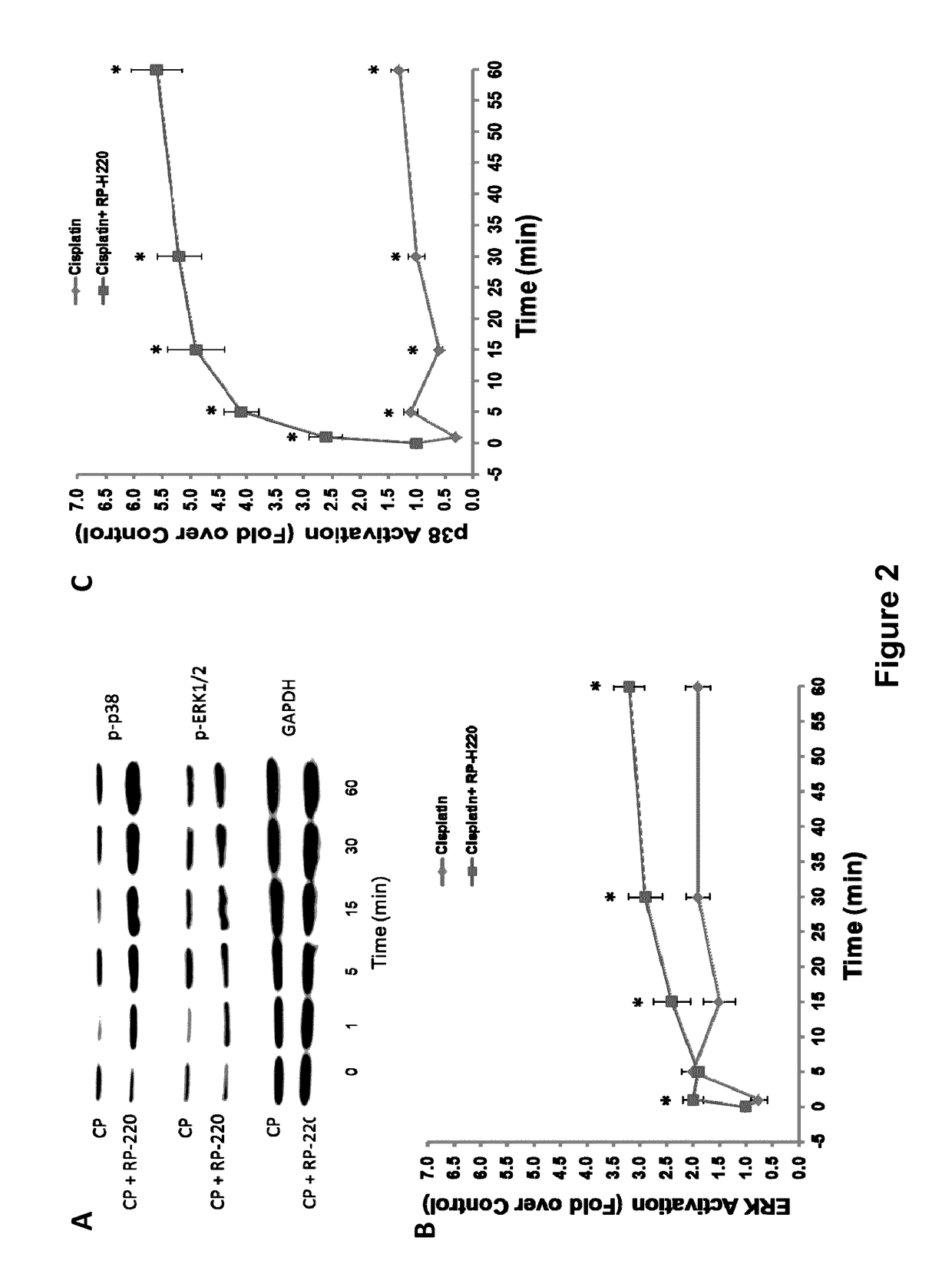
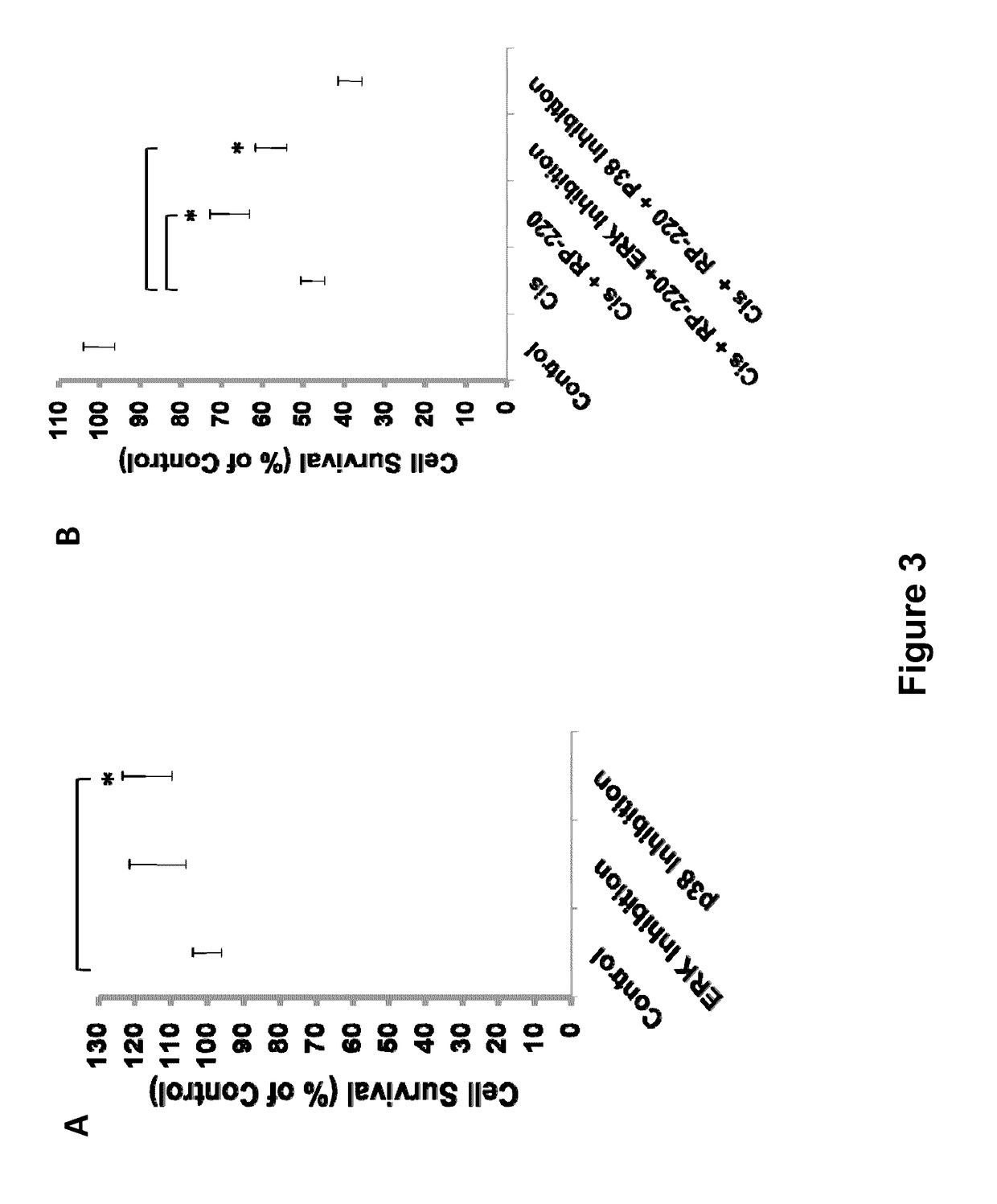
[0229]The results described herein identify PMCA4b as a renalase receptor, and a key mediator of renalase dependent MAPK signaling. Using biotin transfer studies with RP-220 in the human proximal tubular cell line HK-2 and protein identification by mass spectrometry, PMCA4b was identified as a renalase binding protein. This previously characterized plasma membrane ATPase is involved in cell signaling and cardiac hypertrophy. Co-immunoprecipitation and co-immunolocalization confirmed protein-protein interaction between endogenous renalase and PMCA4b. Down-regulation of endogenous PMCA4b expression by siRNA transfection, or inhibition of its enzymatic activity by the specific peptide inhibitor caloxin1b each abrogated RP-220 dependent MAPK signaling and cytoprotection. In control studies, these maneuvers had no effect on epidermal growth factor mediated signaling, confirming specificity of the interaction between PMCA4b and renala...
example 2
Modulation of the Activity of PMCA4b Mediates Renalase's Cytoprotective Action
[0253]The effect of renalase on the ATPase activity of PMCA4b is examined, including its effect on Vmax, Km, and constitutive activation. The local and / or global effect of renalase's interaction with PMCA4b on calcium dynamics is also examined. Whether the disruption of the PMCA4b macromolecular complex with RAS SF-1 modulates the action of renalase (MAPK signaling and cytoprotection) is further examined. Whether PMCA4b knockout mice respond differently than wild type mice to renal ischemia or exposure to cisplatin is examined, as is whether renalase modifies the extent of renal injury.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Level | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com