Embolus Removal Device with Blood Flow Restriction and Related Methods
a technology of embolism and removal device, which is applied in the field of clot retrieval and removal devices, can solve the problems of increased risk of bleeding, cumbersome delivery and deployment, and limited use of intravenous t-pa
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
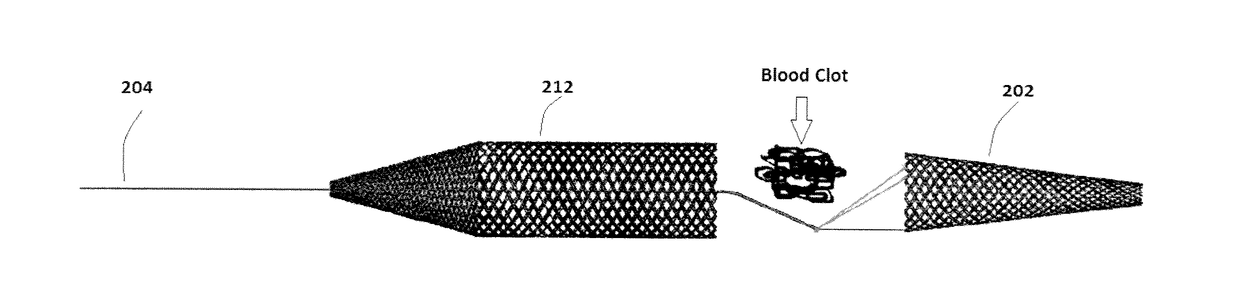
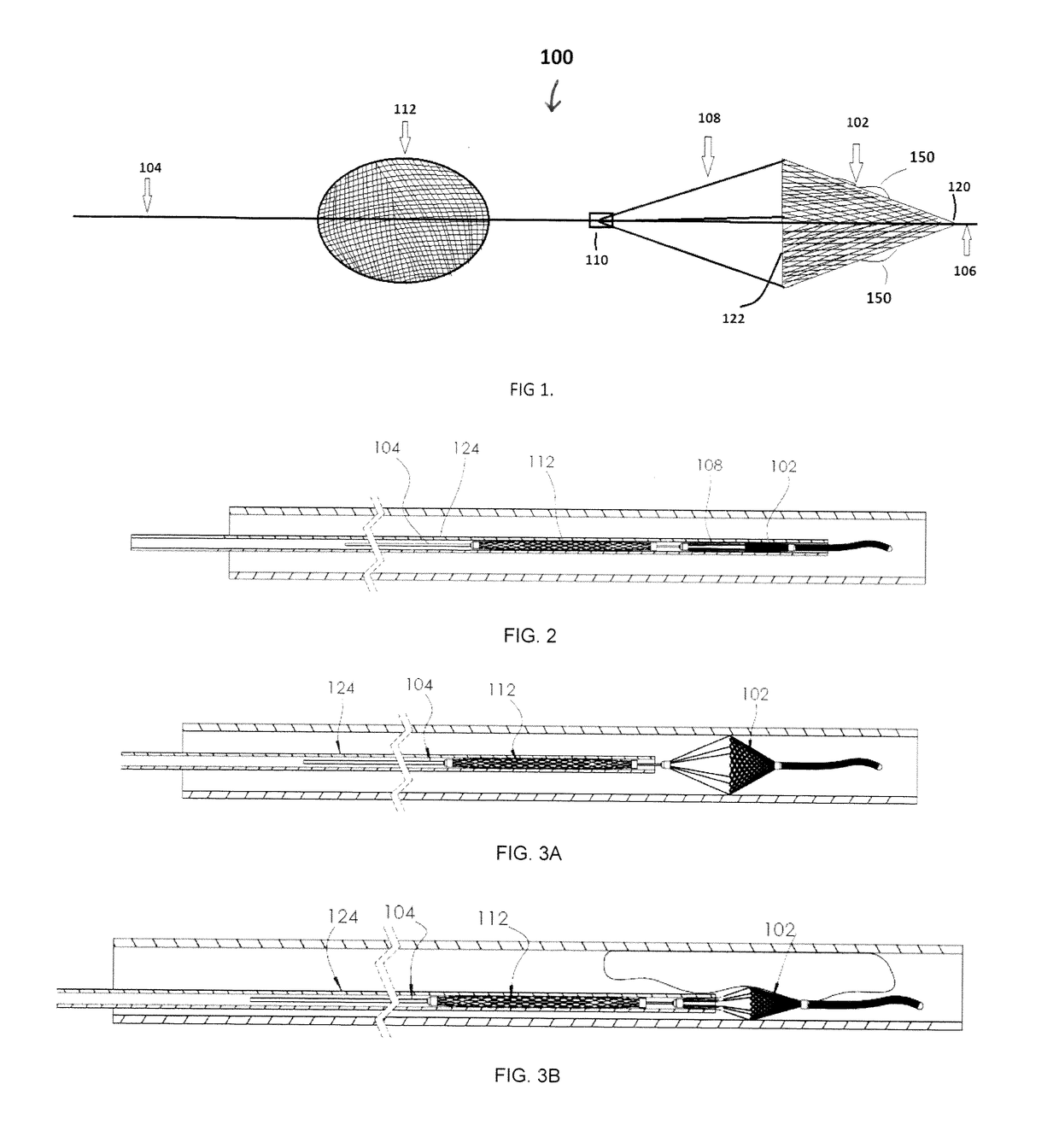
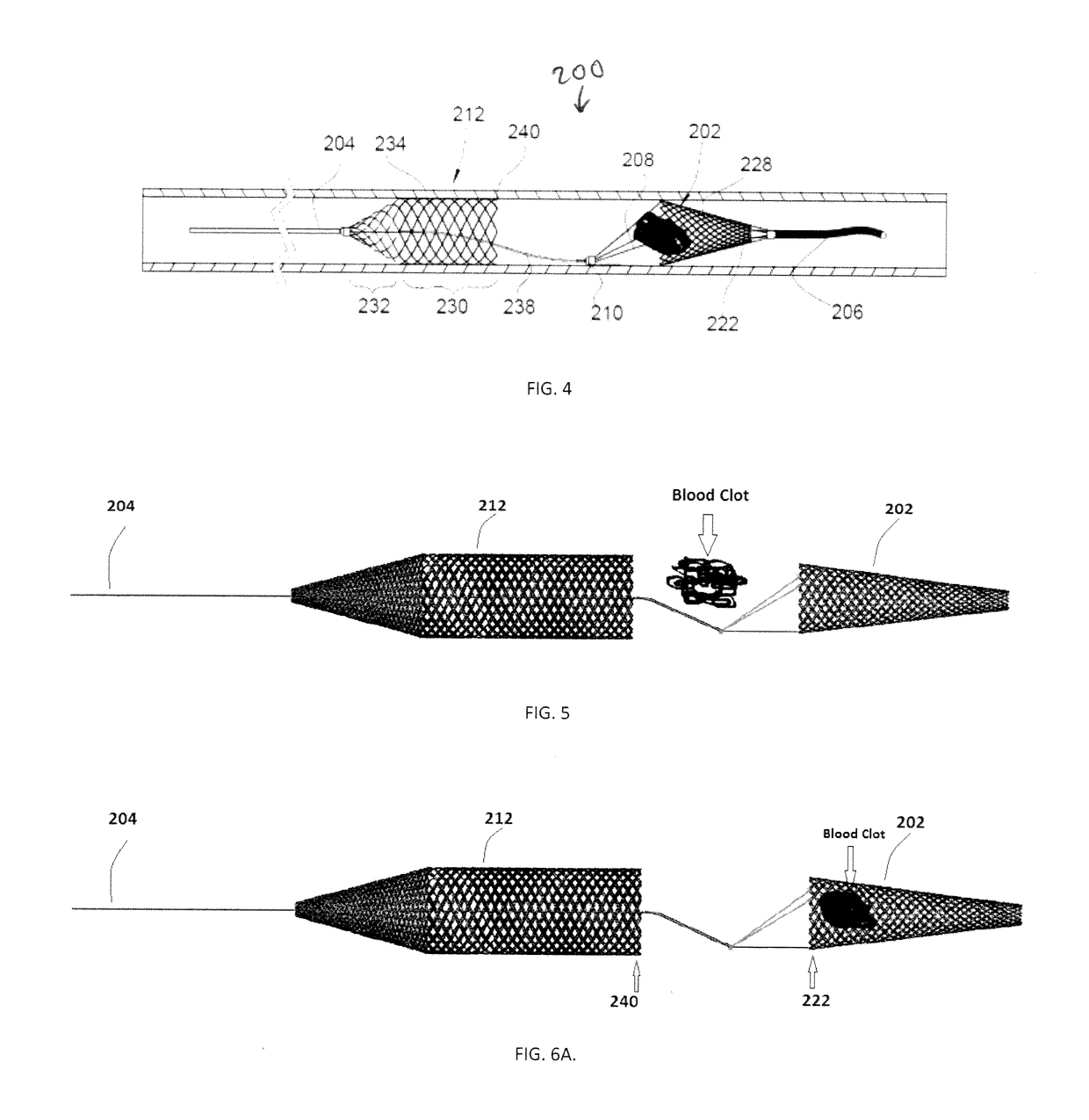
[0063]For example, FIGS. 4-6 illustrate another embodiment of a device 200 for removing emboli and other luminal blockages. The device 200 also has an expandable treatment member 202, a soft distal tip 206 (with marked coil), a delivery wire 204, control arms 208, a hub 210 and a proximal flow restrictor 212 that correspond to the expandable treatment member 102, soft distal tip 106 (with marked coil), delivery wire 104, control arms 108, hub 110 and proximal flow restrictor 112, respectively, for the first embodiment, except for a few differences.
[0064]First, the expandable treatment member 202 has a slightly different configuration. Instead of the conical configuration of the expandable treatment member 102, the expandable treatment member 202 has a frusto-conical body 228 where its distal-most end does not terminate in an apex, but has a small distal opening.
[0065]Second, the proximal flow restrictor 212 has a different configuration, having a body that includes a cylindrical dis...
second embodiment
[0072]FIGS. 7-9 illustrate another embodiment of a device 300 for removing emboli and other luminal blockages. The device 300 is similar to the device 200 in that it also has an expandable treatment member 302, a delivery wire 304, a hub 310 and a proximal flow restrictor 312 that correspond to the expandable treatment member 202, delivery wire 204, hub 210 and proximal flow restrictor 212, respectively, for the second embodiment, except for a few differences.
[0073]First, the expandable treatment member 302 has a different configuration, and can be configured as any of the removal devices disclosed in co-pending United States Publication No. 2015-0150672, filed Jan. 16, 2015, whose entire disclosure is incorporated by this reference as if set forth fully herein. For this reason, there are no control wires 108 / 208.
[0074]Second, the proximal flow restrictor 312 can be essentially the same as the proximal flow restrictor 212 in FIGS. 4-6.
[0075]Third, the hub 310 can function as a marke...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com