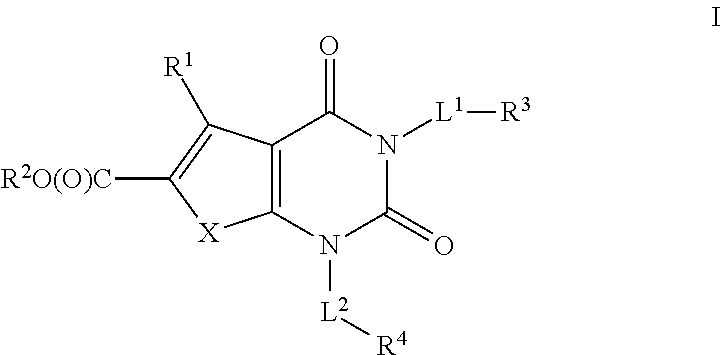
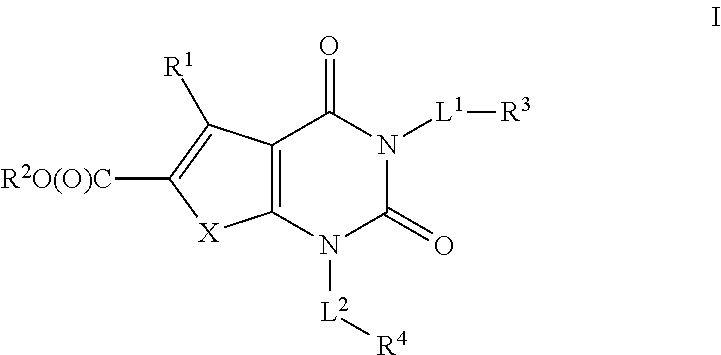
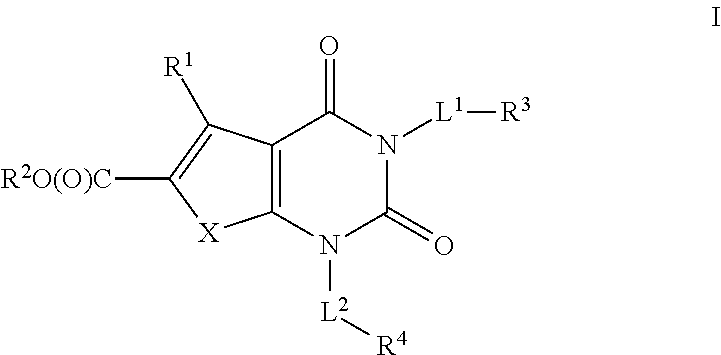
Ester acc inhibitors and uses thereof
a technology of ester acc and inhibitors, which is applied in the direction of antibacterial agents, drug compositions, metabolic disorders, etc., can solve the problems of not providing the long-term health benefit needed for sustainable weight loss, t2dm, stroke and coronary heart disease, and dramatic reduction in quality of li
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
of ethyl (R)-1-(2-(2-methoxyphenyl)-2-((tetrahydro-2H-pyran-4-yl)oxy)ethyl)-5-methyl-3-(2-methyl-1-oxo-1-(pyrrolidin-1-yl)propan-2-yl)-2,4-dioxo-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidine-6-carboxylate, I-3
[0429]
[0430]Synthesis of Compound 1.2.
[0431]Into a 3000-mL 3-necked round-bottom flask under nitrogen, was placed DMSO (1400 mL). This was followed by the addition of NaH (25.0 g, 625.0 mmol, 1.22 equiv, 60%). The mixture was stirred for 15 min at room temperature. To this was added trimethylsulfoxonium iodide (136.0 g, 617.9 mmol, 1.2 equiv.), in portions at room temperature over 30 min. The resulting solution was stirred for 3 h at 40° C. To the mixture was added compound 1.1 (70.0 g, 514.2 mmol, 1.00 equiv) dropwise with stirring at 15° C. The reaction was stirred for 3 hours at room temperature, then quenched by the addition of 1500 mL of NH4Cl (aq.). The resulting solution was extracted with 2×1000 mL of EtOAc, and the organic layers were combined, dried over anhydrous Na2SO4 ...
example 2
of ethyl (R)-3-(1-(ethylamino)-2-methyl-1-oxopropan-2-yl)-1-(2-(2-methoxyphenyl)-2-((tetrahydro-2H-pyran-4-yl)oxy)ethyl)-5-methyl-2,4-dioxo-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidine-6-carboxylate, I-1
[0456]
[0457]Compound I-1 was synthesized from compound 1.95 and ethylamine hydrochloride using procedure described in Example 1. LC-MS (ES, m / z): [M-C2H6N]+ 557, [M+Na]+ 624; 1H NMR (400 MHz, DMSO-d6): δ 0.95-1.05 (t, 3H), 1.15-1.30 (m, 1H), 1.33-1.35 (t, 3H), 1.64-1.67 (m, 8H), 2.74 (s, 3H), 3.05-3.08 (m, 2H), 3.24-3.33 (m, 3H), 3.48-3.65 (m, 2H), 3.80 (s, 3H), 3.90-4.10 (m, 2H), 4.29-4.34 (m, 2H), 5.26-5.29 (t, 1H), 7.00-7.07 (m, 2H), 7.33-7.35 (m, 1H), 7.48-7.55 (m, 2H).
example 3
of ethyl (R)-1-(2-(2-methoxyphenyl)-2-((tetrahydro-2H-pyran-4-yl)oxy)ethyl)-5-methyl-3-(2-methyl-1-oxo-1-(piperidin-1-yl)propan-2-yl)-2,4-dioxo-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidine-6-carboxylate, I-2
[0458]
[0459]Compound I-2 was synthesized from compound 1.95 and piperidine using procedure described in Example 1. LC-MS (ES, m / z): [M-C5H10N]+ 557; 1H NMR (400 MHz, CD3OD): δ 1.35-1.42 (m, 6H), 1.46-1.82 (m, 13H), δ 2.80 (s, 3H), 3.33-3.81 (m, 9H), 3.88 (s, 3H), 4.00-4.25 (m, 1H), 4.34-4.40 (m, 2H), 5.40 (t, 1H), 7.02-7.06 (m, 2H), 7.31-7.35 (m, 1H), 7.51-7.52 (m, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com