Enhancement of glucose-stimulated insulin secretion by cells through induction of cellular senescence
a technology of senescence and glucose stimulation, which is applied in the field of induction of cellular senescence, can solve the problems of reducing the proliferative capacity of -cells, aging tissues typically display, and deteriorating overall function,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
es Cell-Cycle Arrest and Senescence in Pancreatic β-Cells
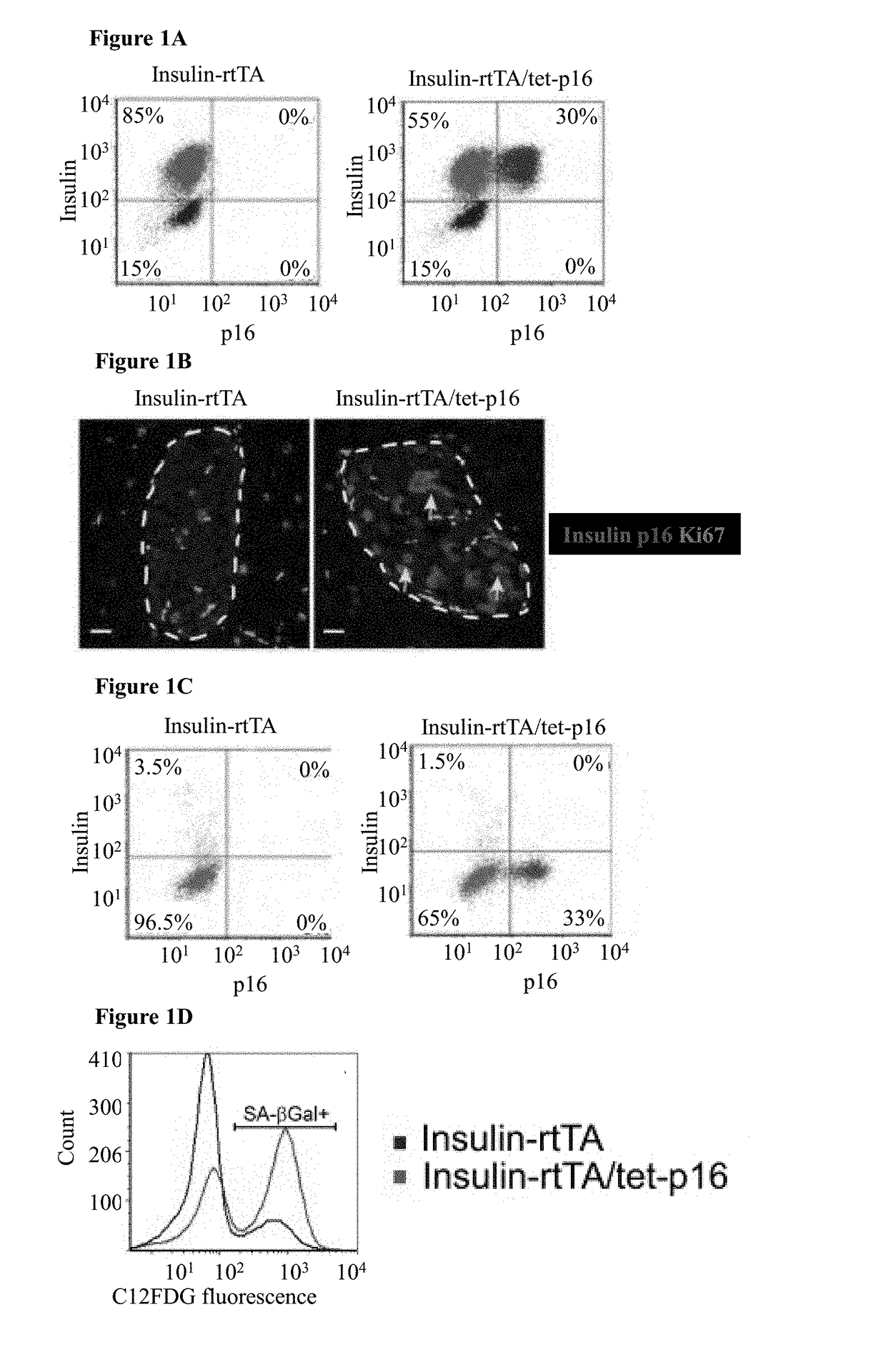
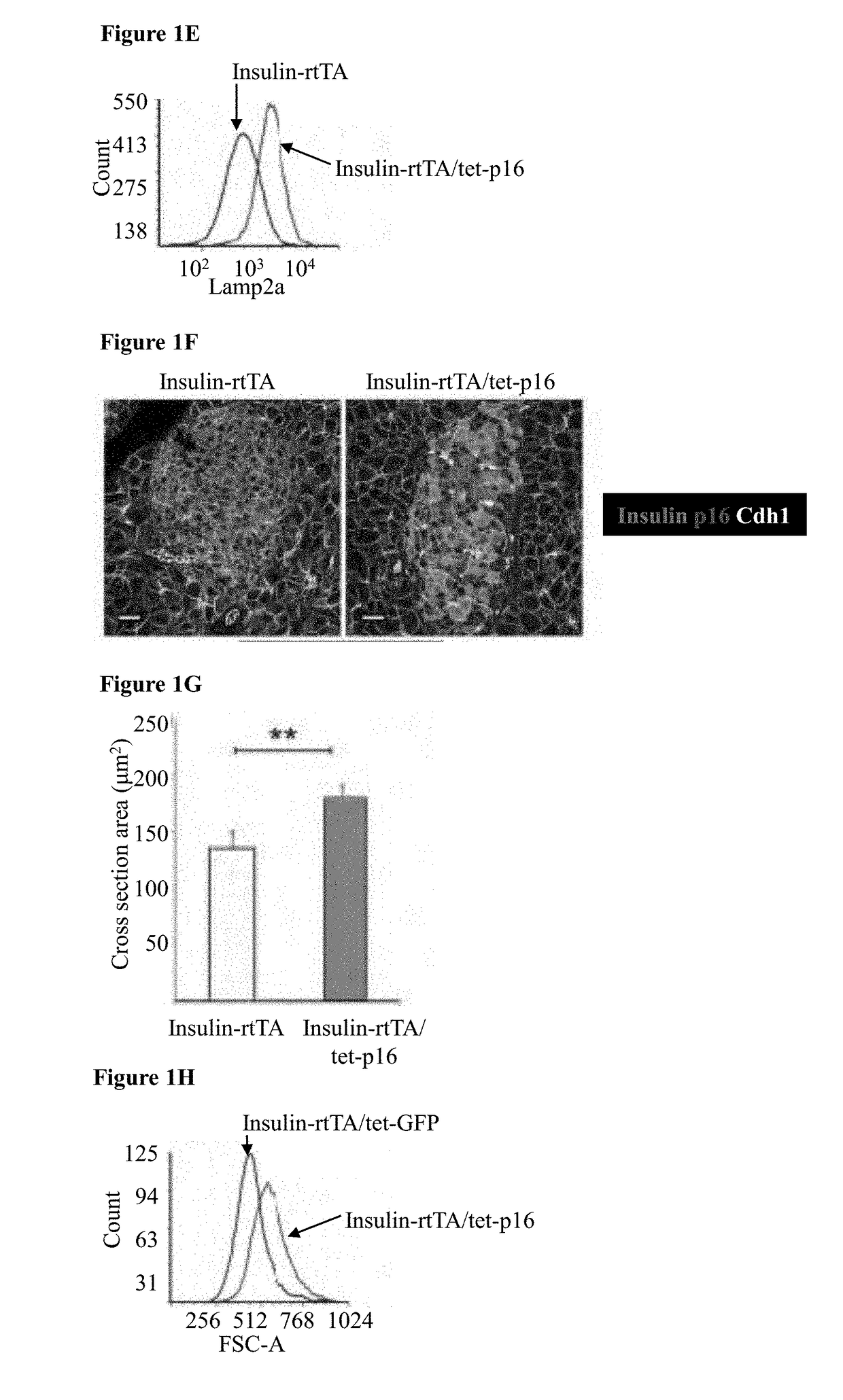
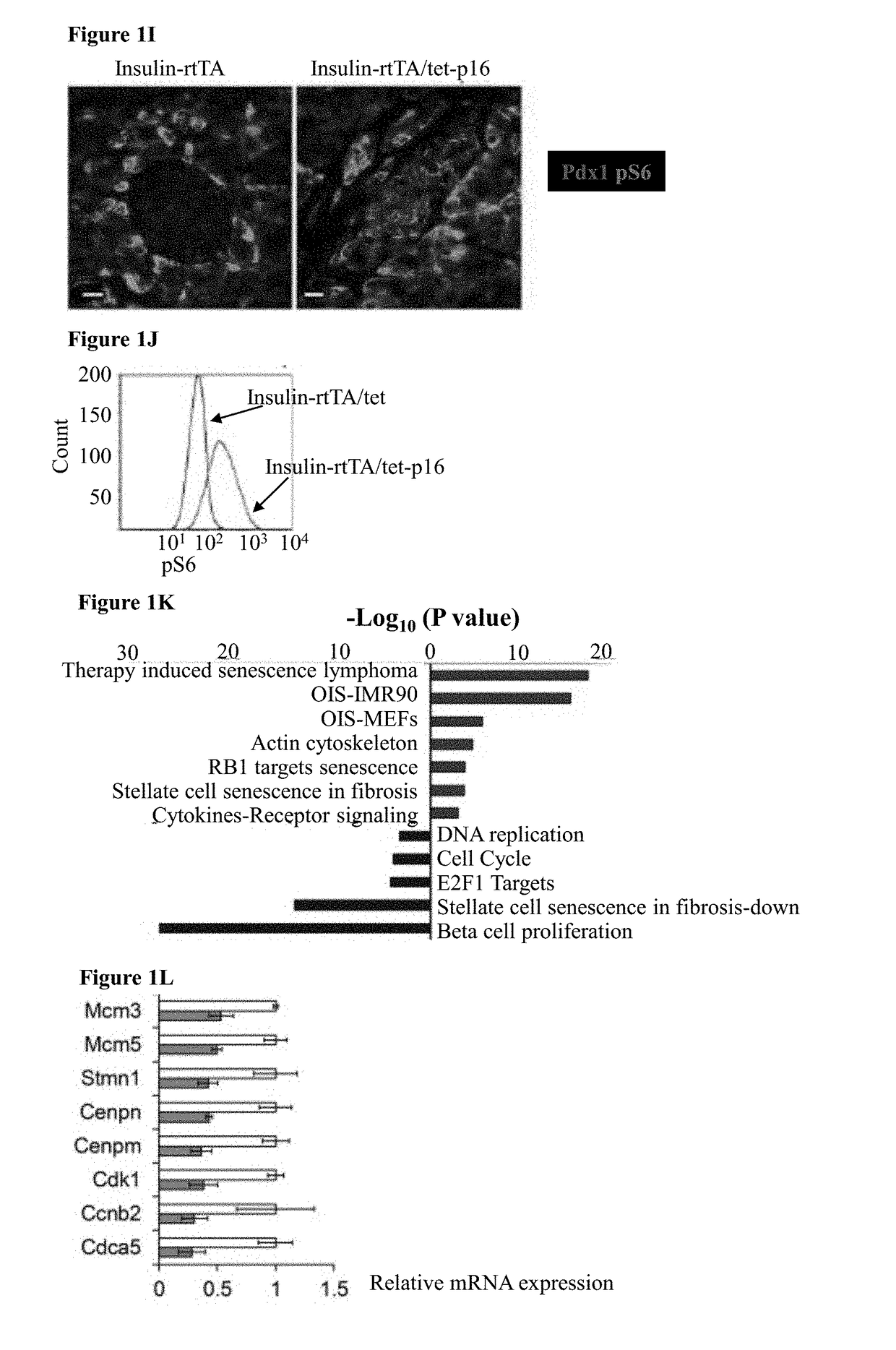
[0152]To study the effects of p16 expression in β-cells mice carrying a tetracycline-inducible human p16 transgene (tet-p16) and crossed them with mice expressing the reverse tetracycline-dependent transactivator under control of the insulin II promoter (Insulin-rtTA) were generated, thus allowing β-cell specific p16 activation upon tetracycline (tet) treatment. In mice treated for 10 days starting at 3-4 weeks of age, transgenic p16 was detected by immunostaining in ˜35% of β-cells; lower-level expression in additional cells could not be excluded (FIGS. 1A-B and FIG. 2A, C). Ki67 staining revealed that 3.5% of β-cells in control mice were proliferating, whereas p16-expressing β-cells were non-proliferative (FIG. 1B-C). Senescence-associated β-galactosidase (SA-βGal) activity and the expression of the lysosomal marker Lamp2a were increased in p16-expressing islets, indicating that p16 induces features of senescence (FIG. 1D-E)...
example 2
ssing β-Cells Express Senescence Genes and Genes Associated with Functional Maturation
[0153]To isolate a population of β-cells enriched for p16 expression, triple-transgenic mice carrying a tet-GFP transgene in addition to tet-p16 and Insulin-rtTA were generated, such that GFP and p16 are co-activated in a largely overlapping subset of β-cells. The mice were treated with tet for 10 days, their islets were dissected and dissociated, and GFP-positive cells were isolated by FACS (FIG. 2C-D). The transcriptome of these cells was compared to that of control β-cells isolated from mice expressing only the GFP transgene (FIG. 2D). It was found that genes associated with cell proliferation were suppressed in p16-expressing β-cells; in contrast, gene sets previously shown to be elevated in senescent cells were significantly upregulated by p16 expression (FIG. 1K-M). Strikingly, these sets were derived from diverse biological settings, including chemotherapy-treated lymphoma cells, Ras-express...
example 3
ases Glucose-Stimulated Insulin Secretion
[0157]To directly study the effect of p16 expression on β-cell function, pancreatic islets were isolated from p16-expressing and control mice following 10 days of tet treatment starting at 4 weeks of age, and measured glucose stimulated insulin secretion (GSIS). While the levels of insulin secreted from islets at low glucose did not significantly differ between p16-expressing and control islets, p16-expressing islets secreted approximately 2.5-fold more insulin upon glucose stimulation (FIG. 5A). This increase was evident when secreted insulin levels were normalized either to total insulin or to protein content in the islet samples (FIG. 5A and FIG. 6A). Normalization of insulin levels directly to β-cell number, scored by FACS, also indicated that secretion per β-cell was elevated (FIG. 5B). GSIS remained high in p16-expressing islets after two months of p16 induction, indicating that this represented a stable phenotypic change (FIG. 5C). Act...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| oxidative stress | aaaaa | aaaaa |
| β-galactosidase activity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com