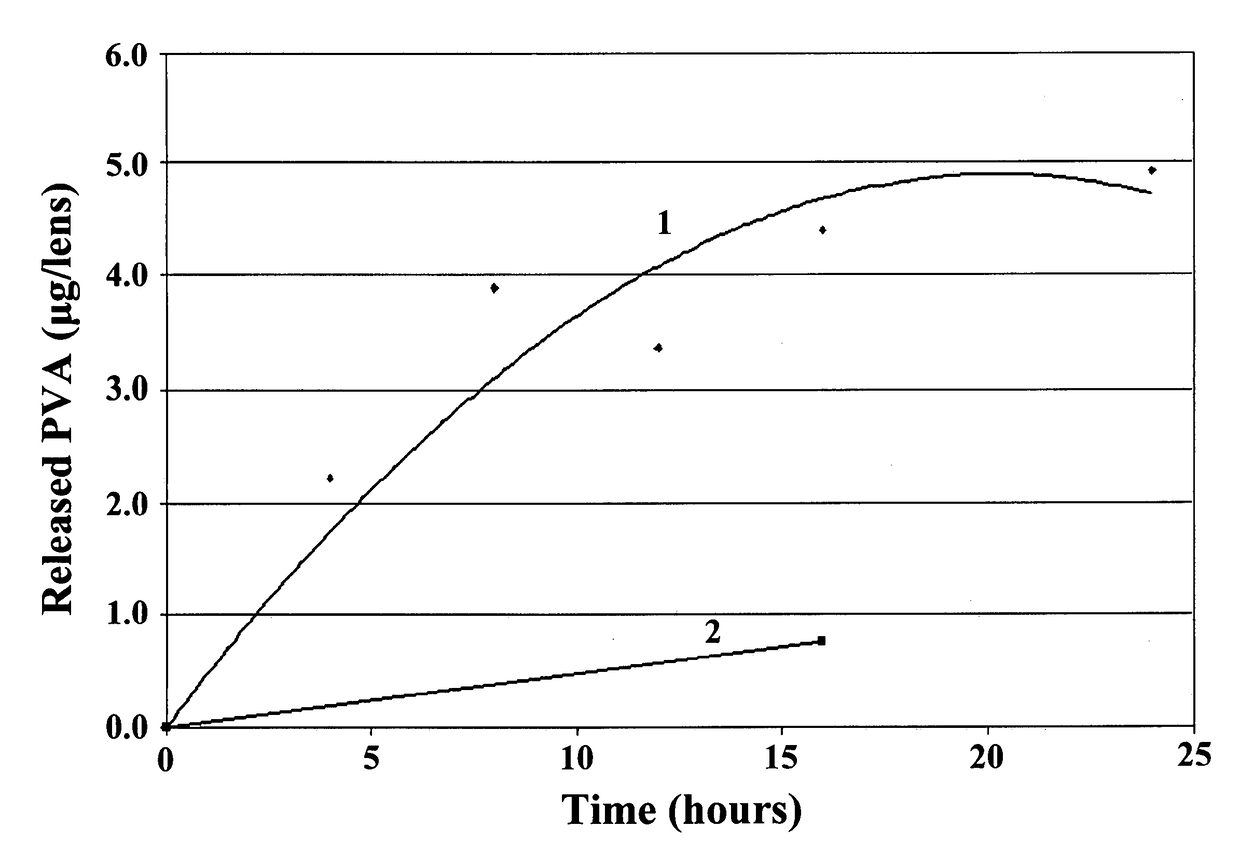
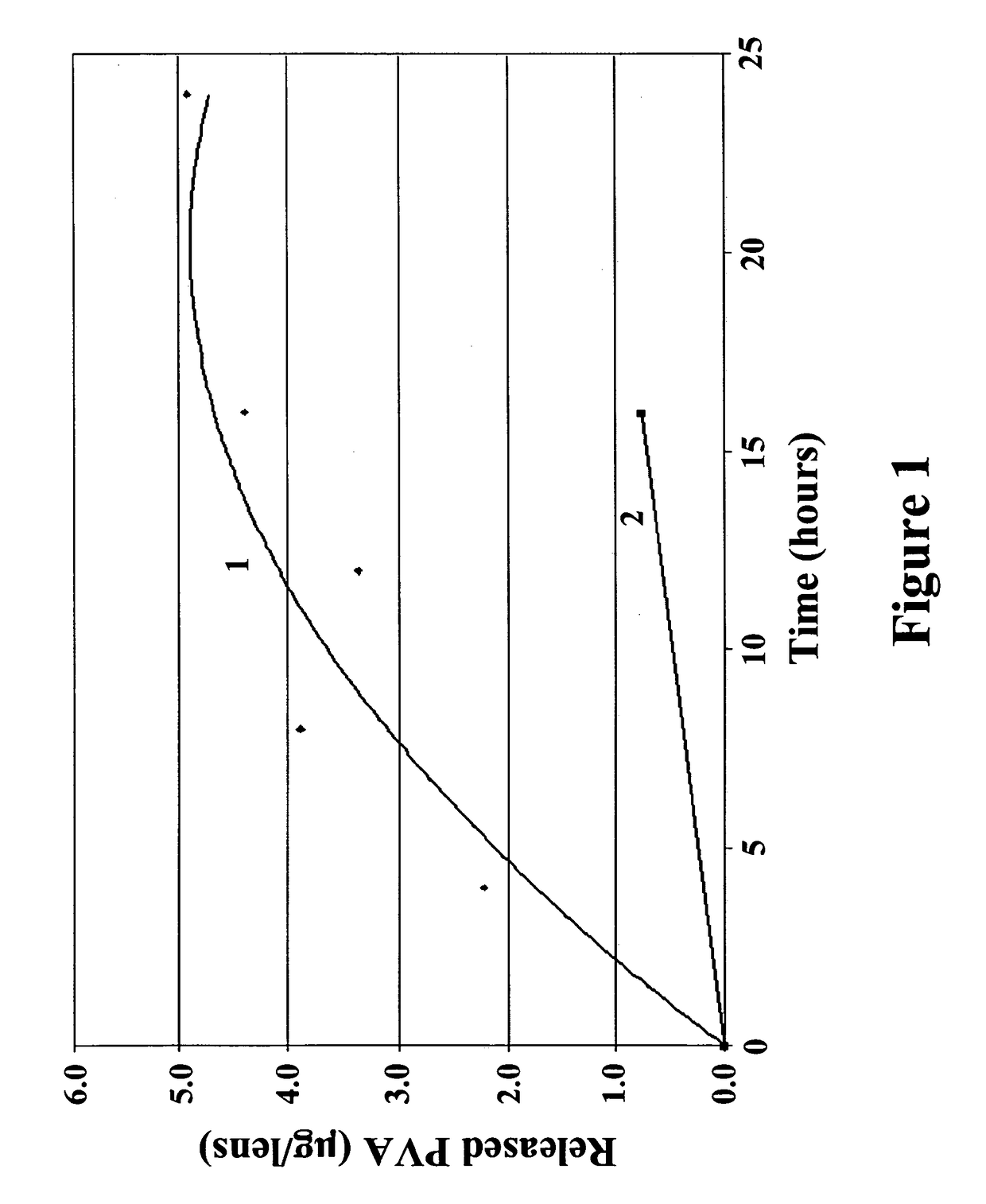
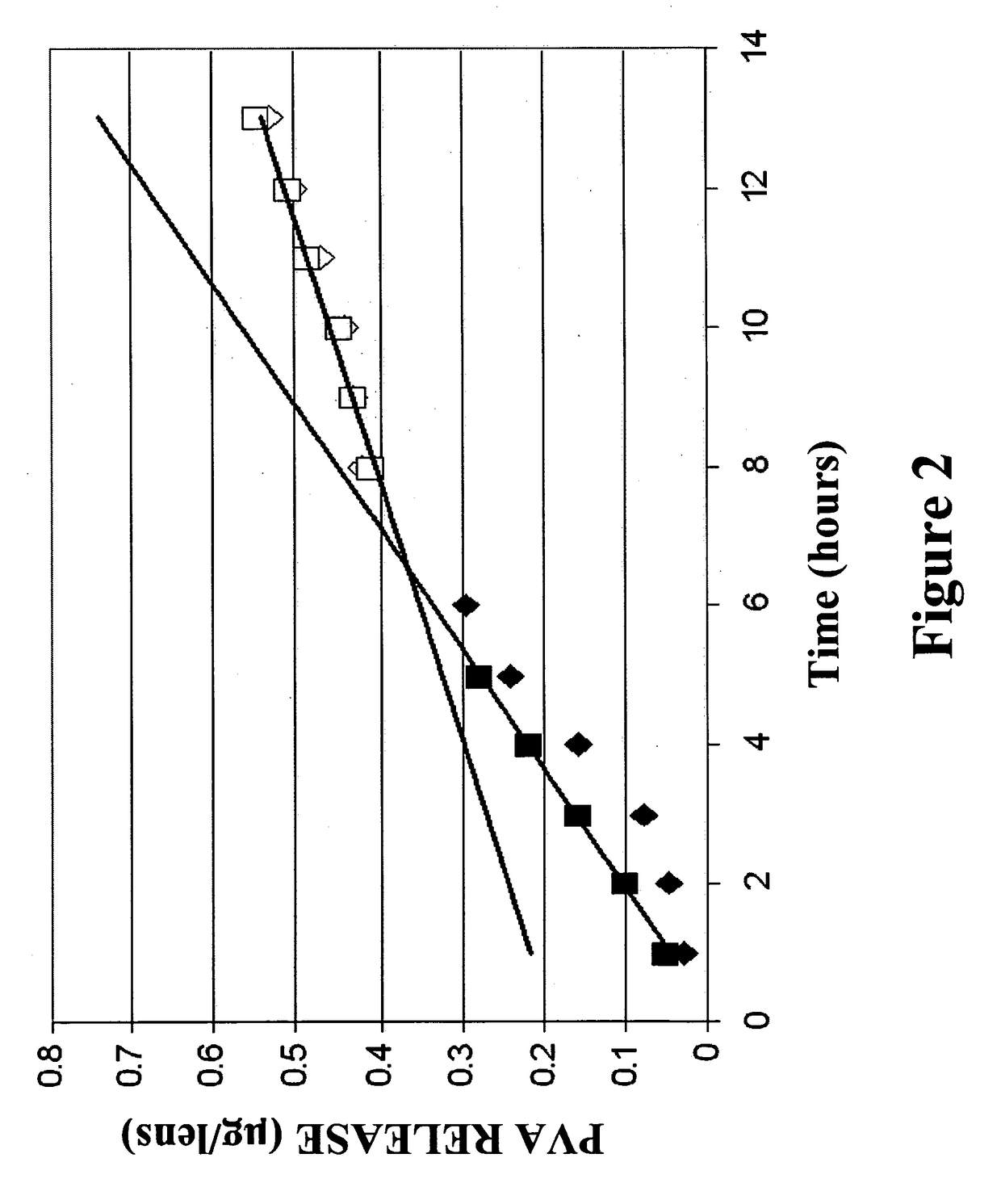
Ophthalmic devices for sustained delivery of active compounds
a technology of active compounds and ophthalmic devices, which is applied in the field of ophthalmic devices for sustained delivery of active compounds, can solve the problems of significant fraction of monomers, unfavorable contact lens industry, and low knowledge of the type of technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0148]Fluid prepolymer compositions (aqueous formulations) are prepared from nelfilcon A (an acrylated-poly(vinyl alcohol) which is water-soluble and actinically-crosslinkable, from CIBA Vision), water, photoinitiator (Irgacure 2959; Ciba Specialty Chemicals), 4-hydroxy-2,2,6,6-tetramethylpiperpiperindinyloxy, free radical (HO-TEMPO; Aldrich Chemicals), poloxamer 108 (Pluronics® F38), non-crosslinkable PVAs (Mowiol 6-98 having Mw ˜47000 from KSE and Mowiol 10-98 having Mw ˜61000 from KSE), and copper phthalocyanine (CuP).
Control Formulation.
[0149]A control formulation is prepared to contain 30% by weight of nelfilcon A, 0.1% by weight of Irgacure 2959, 0.3% by weight of poloxamer 108, and 47 ppm TEMPO.
Formulation I.
[0150]Formulation I is prepared by adding 1% (wt / col) of Mowiol 6-98 and 0.5% Mowiol 10-98 (in a proportion of 3 Mowiol 6-98 to 1 Mowiol 10-98) in the control formulation.
Formulation II.
[0151]Formulation II is prepared from control formulation by adding 1% (wt / col) of Mow...
example 2
Lens Production
[0152]A formulation prepared in Example 1 is dispensed onto a female mold half by using an EFD automatic dispenser (4 bar, 1.2 sec). The female mold half is then mated with a corresponding male mold half. The mold is closed by using a pneumatic closing system. The formulation is UV cured under 2 different UV lights (1.8 mW / cm2 each) for total exposure time of 4.9 sec.
[0153]Each lens is packaged in a conventional blister package containing 0.85 ml phosphate buffered saline and sealed with an aluminum sealing foil. Each lens is autoclaved in the package at 122° C. After autoclaving, the diameter and the E-modulus of the contact lenses are determined. No significant differences in lens diameter and mechanical properties (modulus, elongation, stress, and toughness at break) can be identified between lenses made from the control formulation and formulations I and II.
example 3
[0154]Tests are conducted to evaluate the chemical and physical profile of contact lenses produced in Example 2 under both ambient and accelerated conditions (at 45° C.). For ambient conditions, samples are stored and test at 25° C. at baseline. For accelerated conditions, samples are stored and test at 45°±° C. at 3 and 9 months (equivalent to 12 and 36 months storage at ambient temperature respectively). The stability study follows the guidelines outlined in ISO 11987 for chemical and physical testing required in order to determine the stability of contact lenses and to determine shelf life for these lenses in the blister foil package. There is no significant change in pH and osmolarity of the package saline, light transmittance and percent water content of the lens, power, diameter, and base curve, modulus.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wearing time | aaaaa | aaaaa |
| molecular weights | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com