Tiaprofen acid and isoflavone pharmaceutical composition and application thereof
A technology of tiaprofenic acid and its composition, which is applied in the field of ophthalmic preparations, can solve the problems affecting the efficacy of isoflavones, etc., and achieve the effects of improving permeability, large application prospects, and reducing dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Effect of tiaprofen acid on intraocular permeability of soybean isoflavones
[0030] 1. Method
[0031] 1. Drug preparation: Prepare 5 kinds of liquid medicines with the following final concentrations: (1) 800 μg / mL tiaprofen acid + 80 μg / mL soybean isoflavone liquid (mass ratio 1:0.1); (2) 160 μg / mL thialofen Fenac + 80 μg / mL soybean isoflavone liquid (mass ratio 1:0.5); (3) 80 μg / mL tiaprofen acid + 80 μg / mL soybean isoflavone liquid (mass ratio 1:1); (4) 40 μg / mL tiaprofen acid+80 μg / mL soybean isoflavone liquid (mass ratio 1:2); (5) 32 μg / mL tiaprofen acid+80 μg / mL soybean isoflavone liquid (mass ratio 1:2.5); (6) 80μg / mL soybean isoflavone liquid.
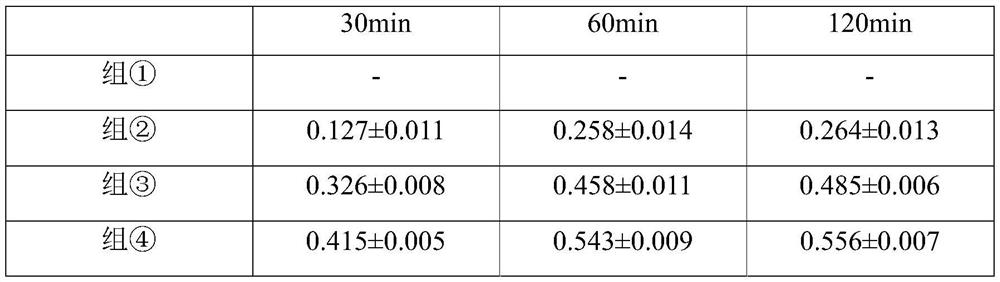
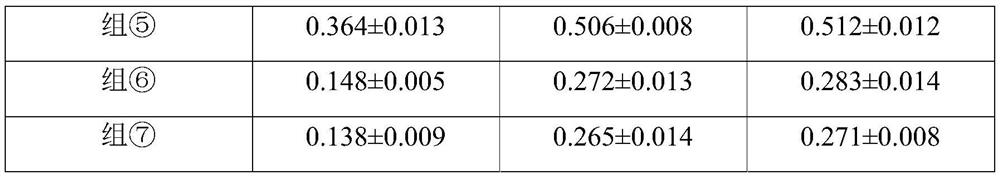
[0032] 2. Experimental animals and grouping: 63 healthy and clean New Zealand white rabbits, weighing 2.0-2.5 kg, half male and half male. The 63 rabbits were randomly divided into 7 groups, 9 in each group: each group was divided into 3 subgroups according to the time points of 30, 60, and 120 minutes aft...
Embodiment 2
[0043] Example 2 Effect of tiaprofen acid on retinal macular inflammation and soybean isoflavone treatment effect
[0044] 1. Method
[0045] Choroidal neovascularization (CNV) model induced by 532nm argon laser was selected from C57 / B6 mice. (The laser point should avoid the main blood vessels and be 2.5 to 3.0 optic disc diameters away from the optic nerve head.) Divide the successfully modeled mice into 7 groups, n=5 in each group; ①The liquid matrix control group (blank control group ), ②800μg / mL tiaprofen acid+80μg / mL soybean isoflavone liquid group, ③160μg / mL tiaprofen acid+80μg / mL soybean isoflavone liquid group, ④80μg / mL tiaprofen acid+80μg / mL soybean Isoflavone liquid group, ⑤80 μg / mL tiaprofen acid + 160 μg / mL soybean isoflavone liquid group, ⑥80 μg / mL tiaprofen acid + 200 μg / mL soybean isoflavone liquid group, ⑦80 μg / mL (0.008%) soybean For the isoflavone drug solution group and (8) 80 μg / mL tiaprofen acid solution group, the volume of 50 μL per eye was locally ad...
Embodiment 3~5
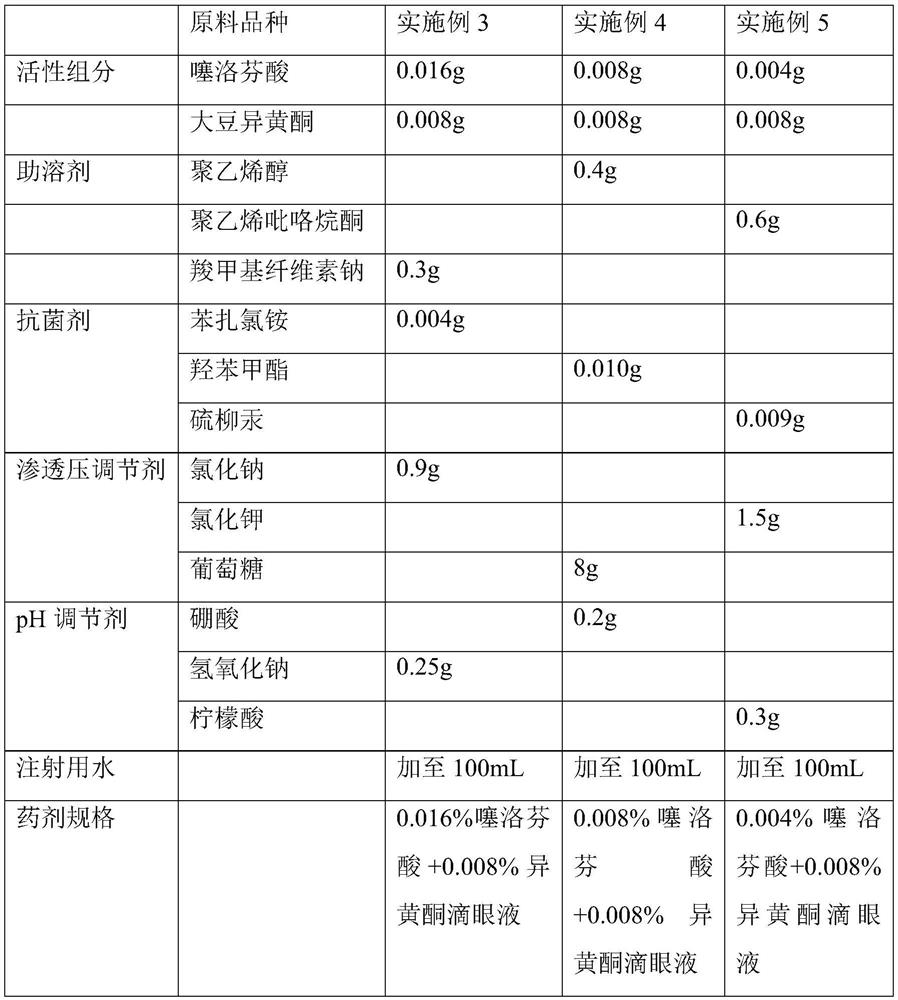
[0053] Prepare tiaprofen acid + soybean isoflavone eye drops with different component contents, as shown in Table 4:
[0054] Table 4 Embodiment 3~5 eye drop formula
[0055]
[0056] According to the technical scheme of the present invention, the optional adjuvant varieties for preparing tiaprofen acid+soybean isoflavone eye drops are not limited to the varieties listed in the above table, and can also have the following multiple options:
[0057] The antibacterial agent is one or more selected from ethylparaben, methylparaben, propylparaben, phenylmercuric acetate, chlorobutanol, thimerosal, benzalkonium chloride and benzalkonium bromide.
[0058] Use an osmotic pressure regulator to adjust the osmolality of the finished eye drops to be 280-330mOsmol / kg; the osmotic pressure regulator is one of sodium chloride, potassium chloride, glucose, boric acid, borax, glycerin, mannitol or Various.
[0059] Use a pH regulator to adjust the pH of the finished eye drops to 5.0 to 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com