Therapeutic and diagnostic methods for cancer
a cancer and diagnostic method technology, applied in the field of cancer diagnostic methods and therapeutic and diagnostic methods, can solve the problems of poor outcomes, difficult timely detection and treatment, and cancer remains one of the most deadly threats to human health, and achieve the effect of inhibiting the binding of pd-l1 and inhibiting the binding of pd-l1
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
tochemical (IHC) Analysis of PD-L1 Expression in Tumor Samples
[0444]Immunohistochemistry (IHC):
[0445]Formalin-fixed, paraffin-embedded tissue sections were deparaffinized prior to antigen retrieval, blocking and incubation with primary anti-PD-L1 antibody. Following incubation with secondary antibody and enzymatic color development, sections were counterstained and dehydrated in series of alcohols and xylenes before coverslipping.
The following protocol was used for IHC. The Ventana Benchmark XT or Benchmark Ultra system was used to perform PD-L1 IHC staining using the following reagents and materials:
Primary antibody: anti-PD-L1 Rabbit Monoclonal Primary Antibody
Specimen Type: Formalin-fixed paraffin embedded (FFPE) section of tumor samples
Epitope Recovery Conditions: Cell Conditioning, standard 1 (CC1, Ventana, cat #950-124)
Primary Antibody Conditions: 1 / 100, 6.5 μg / ml for 16 minutes at 36° C.
Diluent: Antibody dilution buffer (Tris-buffered saline containing carrier protein and BRI...
example 2
on Between PD-L1 Expression in Tumor-Infiltrating Immune Cells (ICs) and Response to Treatment with PD-L1 Axis Binding Antagonists
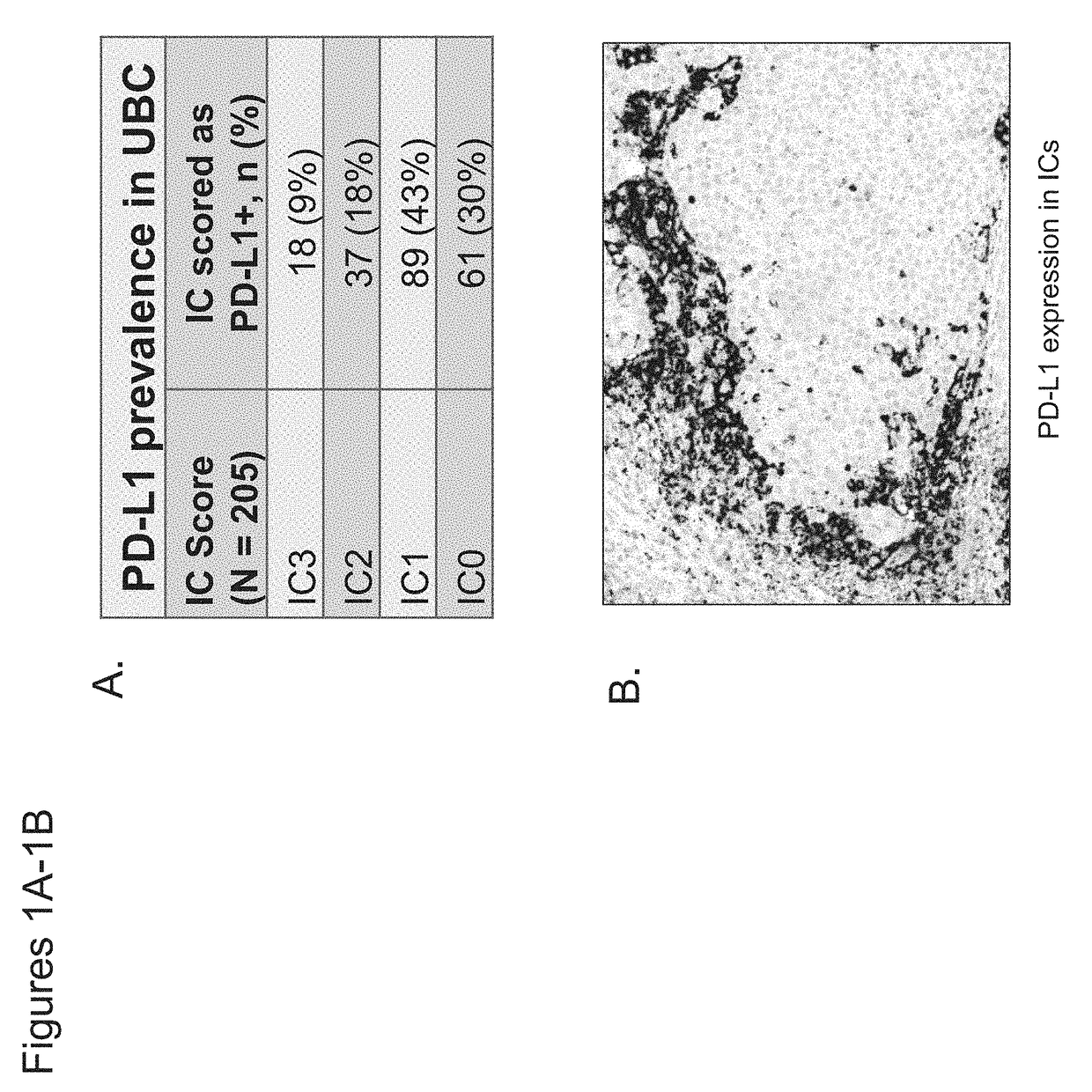
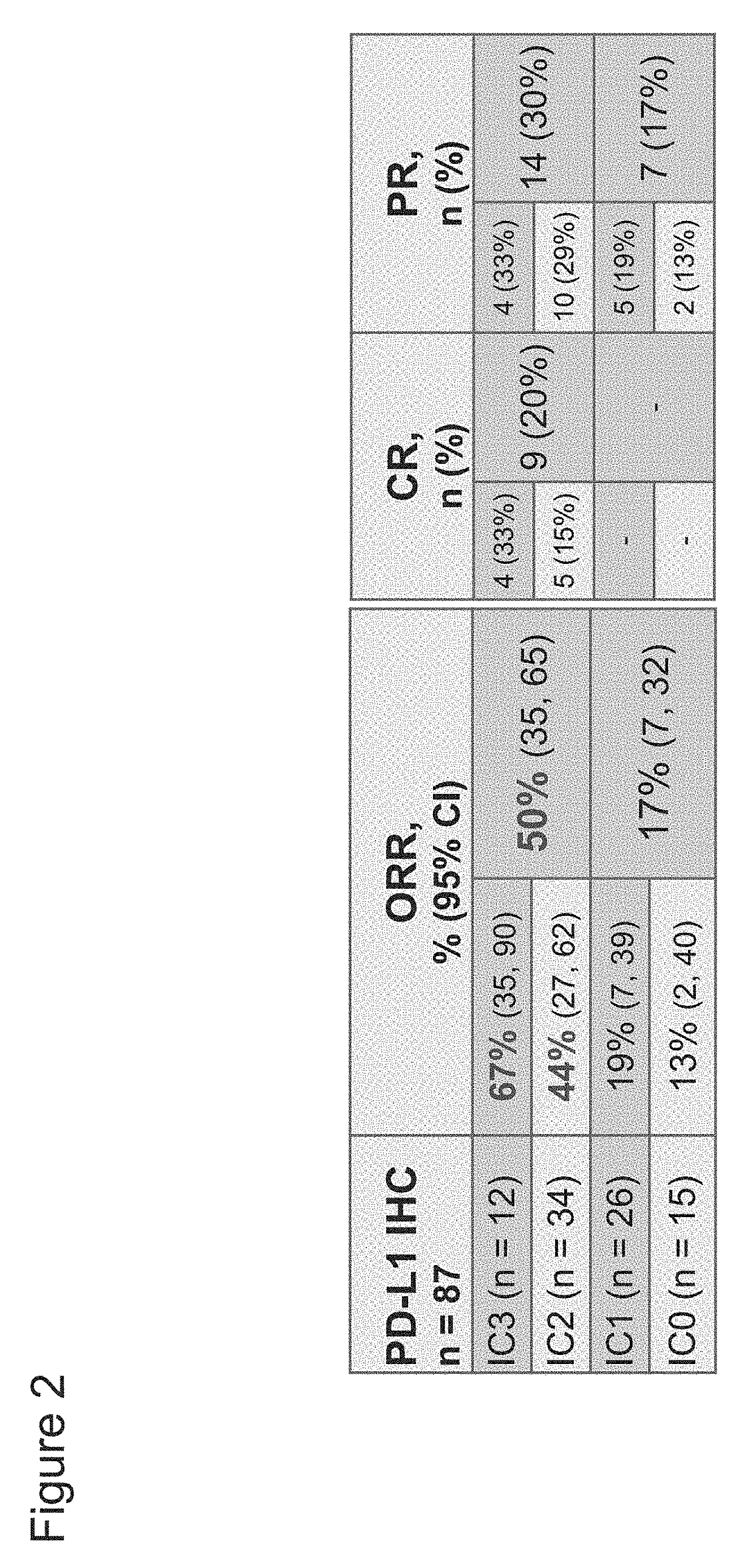
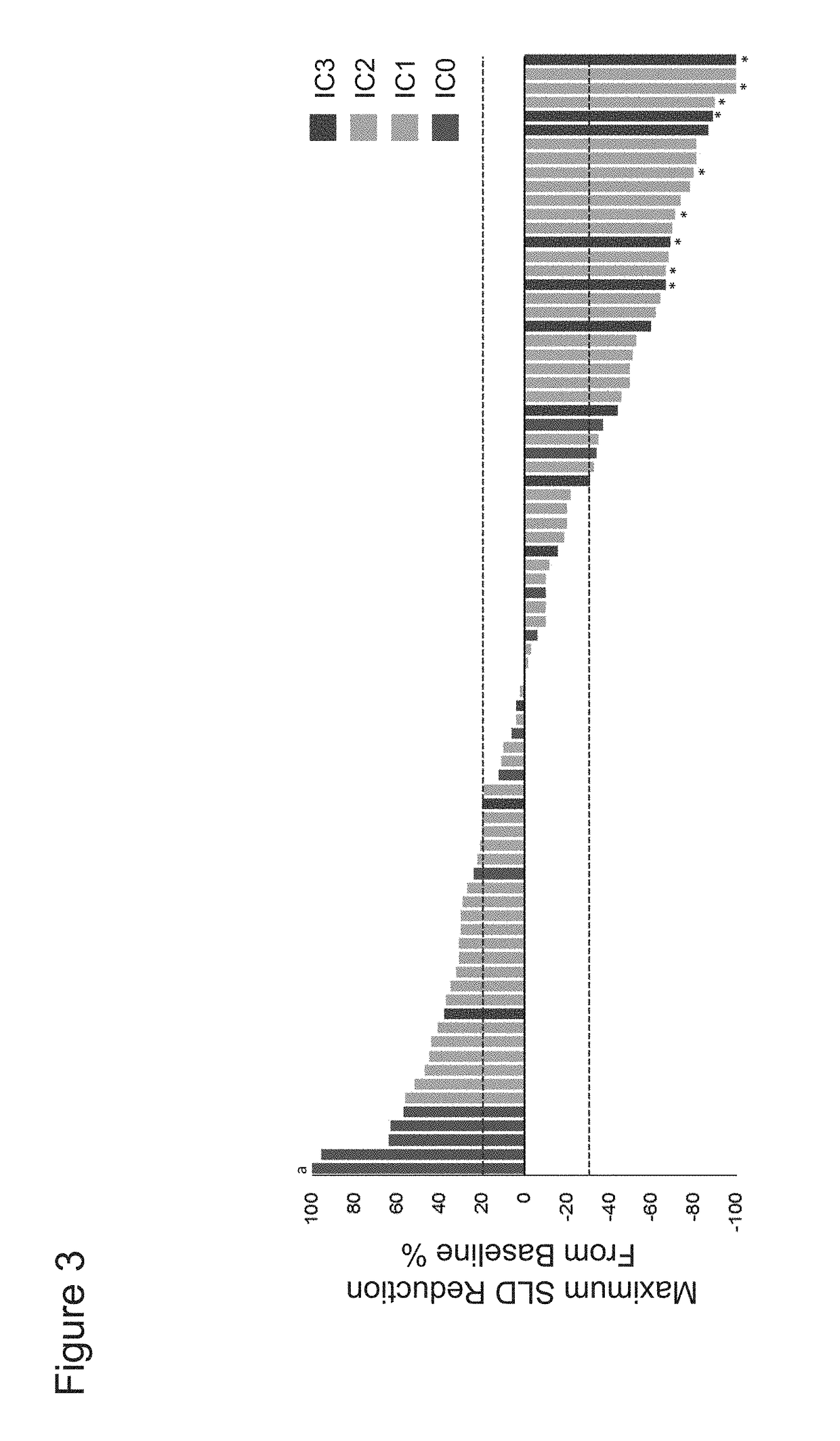
[0449]The association between PD-L1 expression in tumor-infiltrating immune cells within urothelial bladder cancer (UBC) tumors with benefit from treatment with PD-L1 axis binding antagonists was evaluated. The UBC patients studied were enrolled in an ongoing phase Ia study that includes a cohort of UBC patients (safety-evaluable UBC population=92). Key eligibility criteria included measurable disease per Response Evaluation Criteria In Solid Tumors (RECIST) v1.1 and an Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) of 0 or 1. The UBC cohort originally enrolled patients with PD-L1 IC scores of IC2 / 3 but was then expanded to include all-comers, primarily recruiting PD-L1 IC0 / 1 patients. PD-L1 IC scores were scored as shown in Table 3. Atezolizumab (MPDL3280A) was administered intravenously (IV) every three weeks (q3w) at 15 mg / kg or 1200...
example 3
Study Examining the Association of Immunoblocker Signature and CTLA4 Expression Levels on Therapy with Response of UBC Patients to Atezolizumab
[0455]The association between response to treatment with atezolizimab with expression of a “immunoblocker” signature (including the genes CTLA4, BTLA, LAG3, HAVCR2, and PD1) during therapy was evaluated during the course of a Phase Ia clinical study that included a cohort of UBC patients.
[0456]As shown in FIG. 6, increased mRNA expression (as determined by a custom Nanostring assay) of the immunoblocker signature, as well as CTLA4, by T-cells by cycle 3, day 1 of treatment was associated with response to atezolizumab in UBC patients. Therefore, the expression levels of CTLA4, BTLA, LAG3, HAVCR2, and PD1 represent potential biomarkers for response of UBC patients to treatment with PD-L1 axis binding antagonists, including the anti-PD-L1 antibody atezolizumab.
PUM
| Property | Measurement | Unit |
|---|---|---|
| survival time | aaaaa | aaaaa |
| survival time | aaaaa | aaaaa |
| survival time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com