Methods for the treatment and prevention of inflammatory diseases
a technology of inflammatory diseases and glycolipids, applied in the field of methods and glycolipid compounds for the treatment of inflammatory diseases, can solve the problems of disproportionately affecting children, bronchial asthma, 10% of the general population, etc., and achieve the effects of suppressing airway hyperreactivity, suppressing allergen-induced ahr, and inhibiting bal inflammation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Materials and Methods
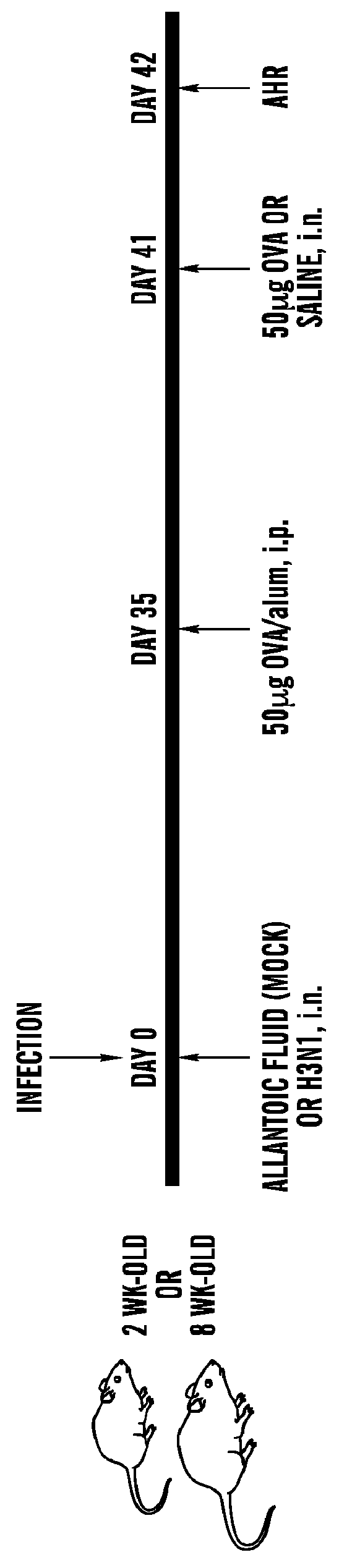
[0376]Mice. Wild-type BALB / c ByJ and T-bet− / − (C.129S6-Tbx21tm1G1m / J) mice were purchased from The Jackson Laboratory. Jα18− / − mice were gifts from M. Taniguchi and T. Nakayama (Chiba University, Chiba, Japan). TLR7− / − mice were generated by Dr. Shizuo Akira (Chiba University, Chiba, Japan), and the Vα14 Tg mice were provided by Dr. Albert Bendelac (University of Chicago, Chicago, Ill., USA). These strains were backcrossed to BALB / c for more than 10 generations. DO11.10×Rag− / − mice were provided by Dr. Abul Abbas (UCSF, San Francisco). For studies in suckling mice, BALB / c, TLR7− / − and T-bet− / − mice were bred, and the offspring were infected at 2 wks of age, then weaned at 3 wks. The Animal Care and Use Committee at Children's Hospital Boston approved all animal protocols.
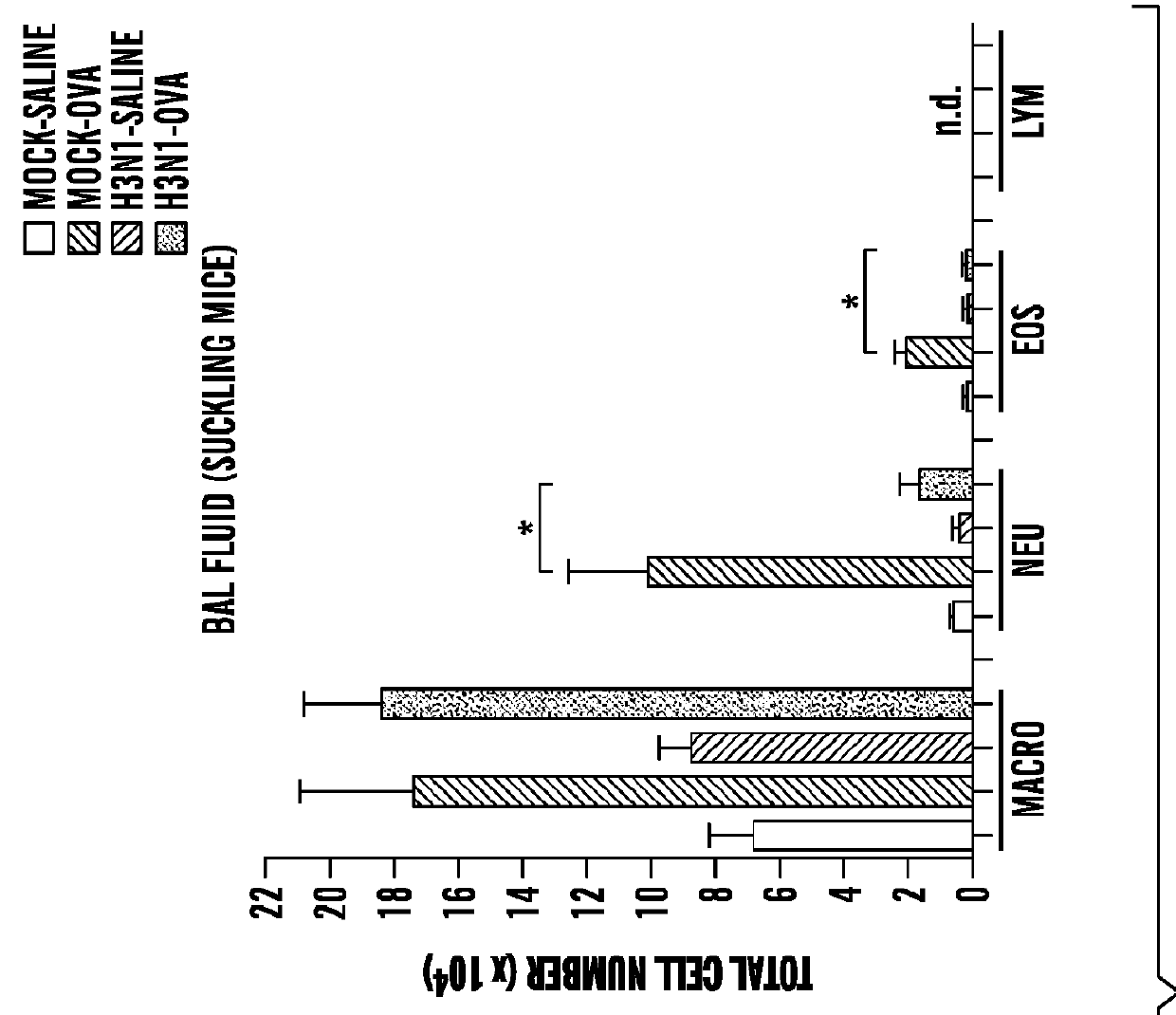
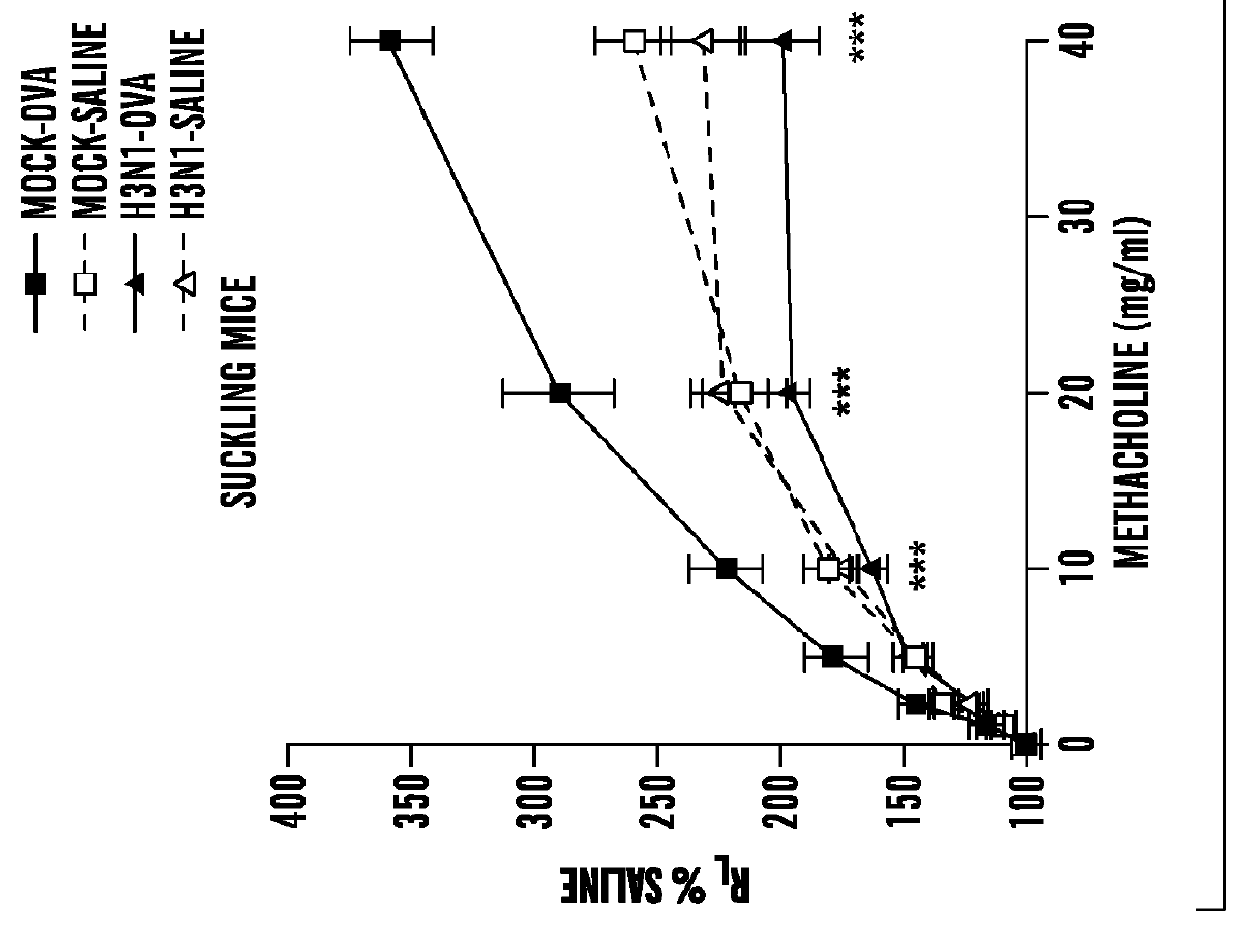
[0377]Influenza A infection. Two-week-old mice (suckling mice) or 8-week-old (adult mice) were anesthetized with 3% isoflurane and inoculated intranasally (i.n.) with influenza A virus (strain M...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mean diameters | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com