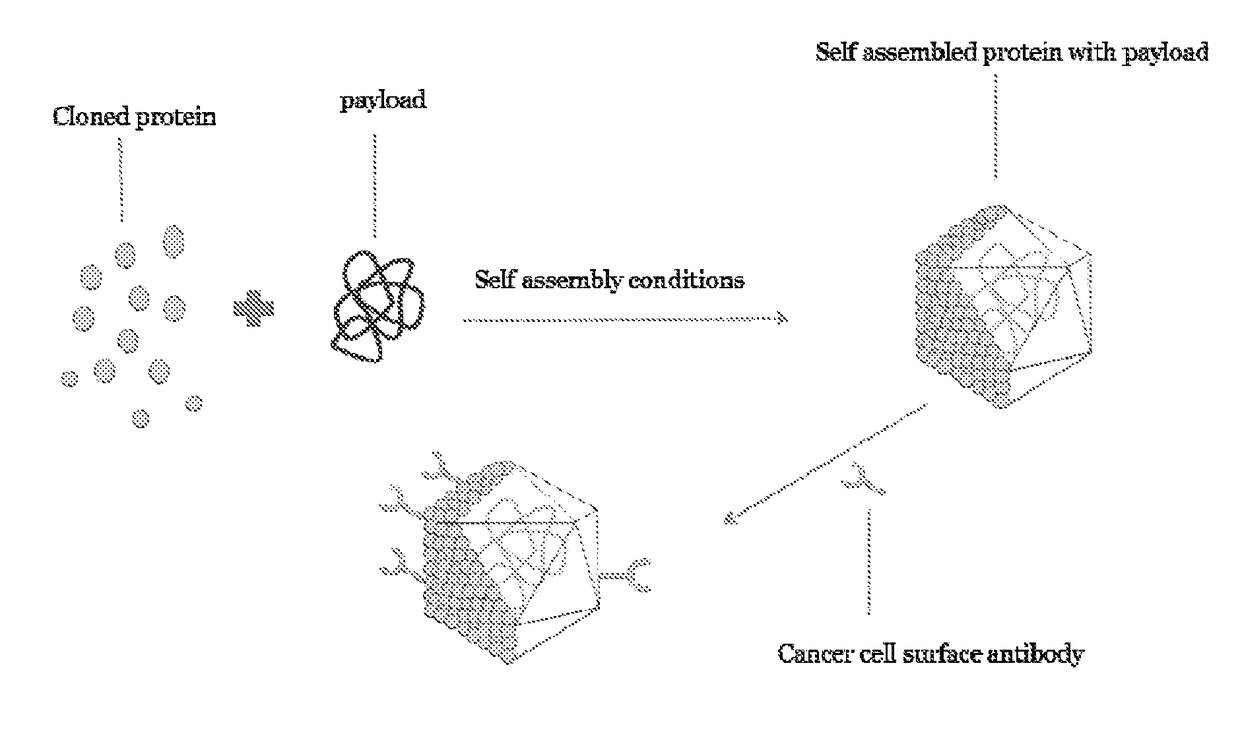
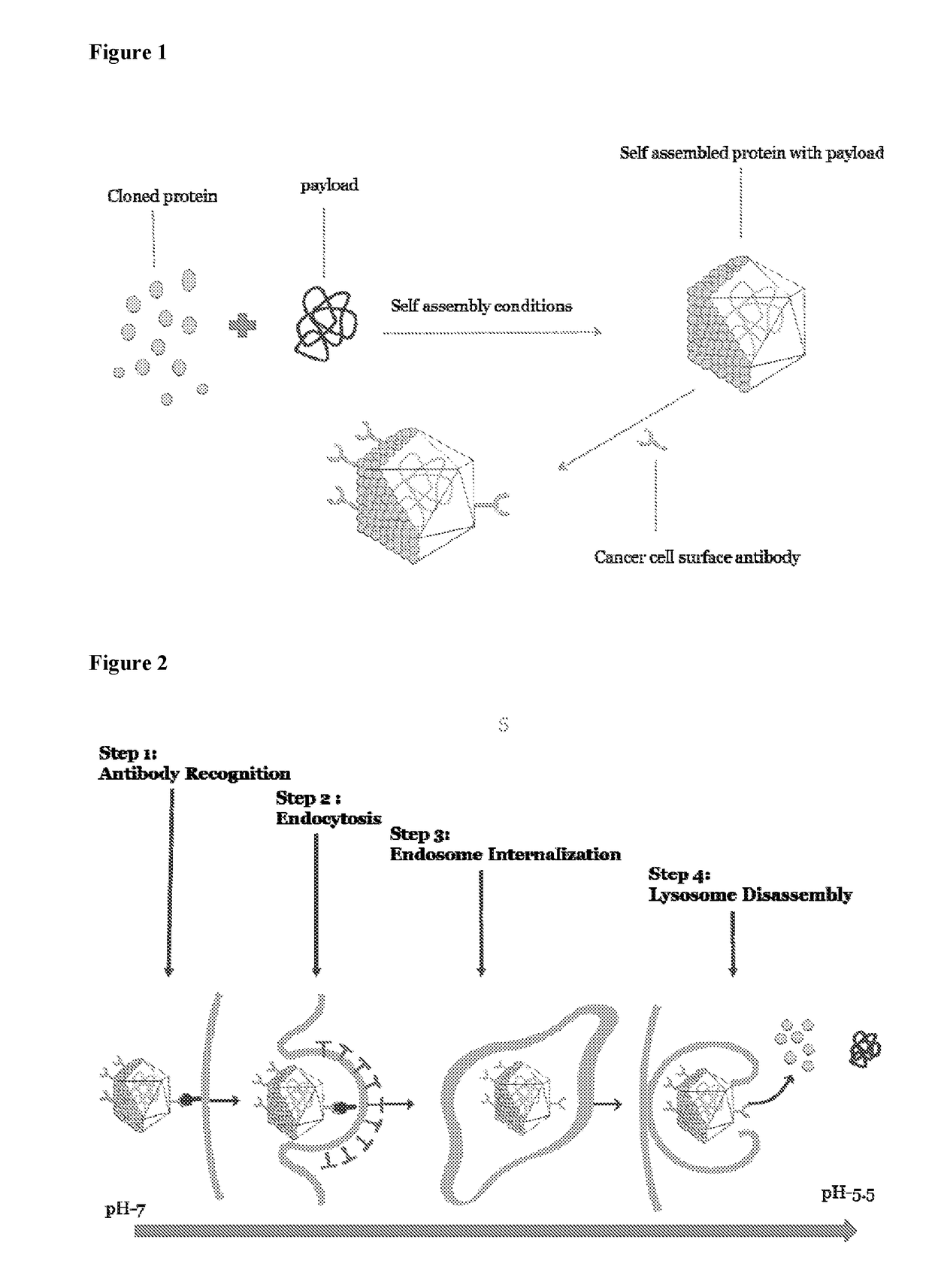
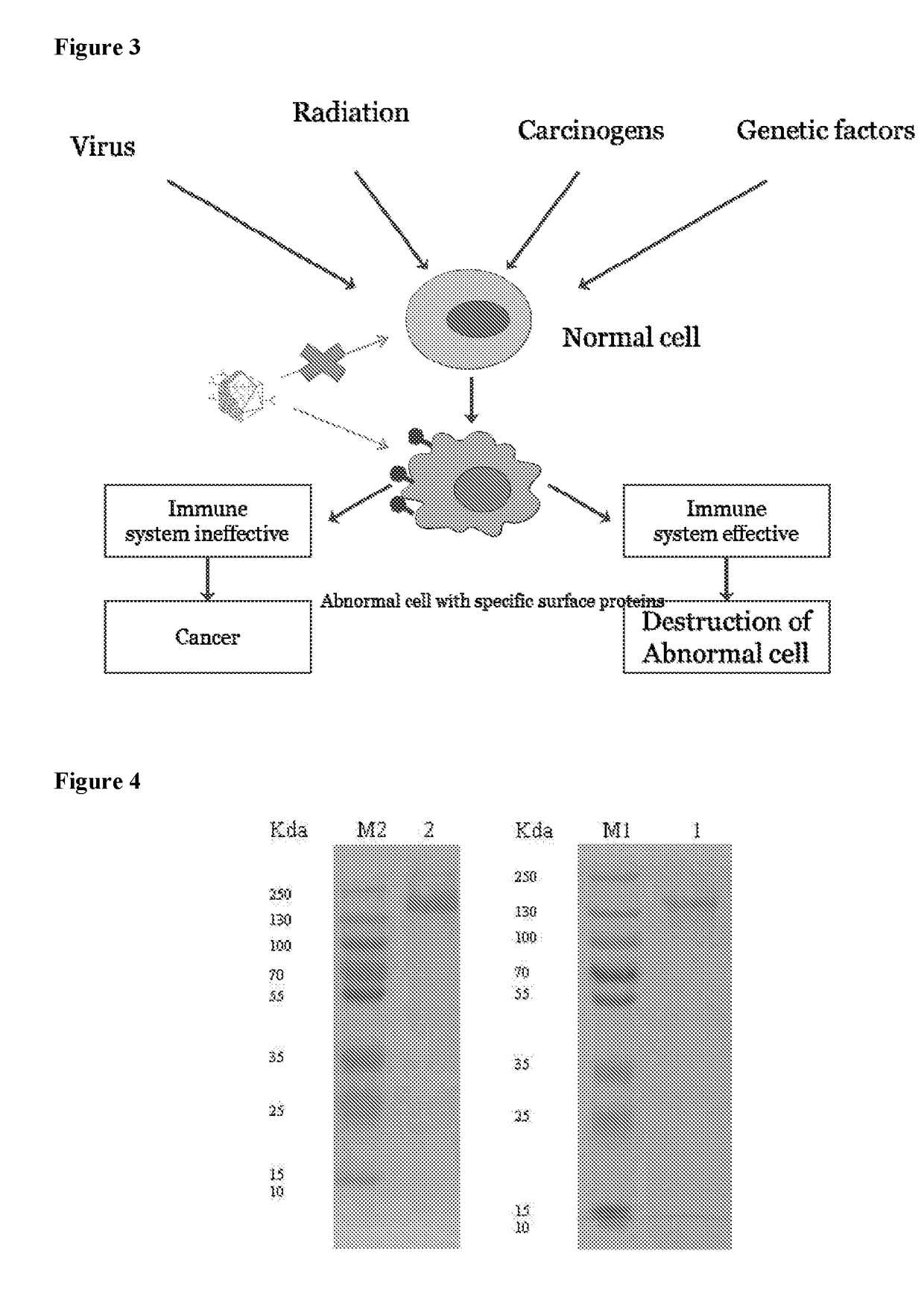
Self assembling molecules for targeted drug delivery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
n of Clathrin Heavy Chain
[0173]Clathrin human isoform 2 heavy chain was optimized for an E. coli expression system as follows:
(SEQ ID NO: 1):MAQILPIRFQEHLQLQNLGINPANIGFSTLTMESDKFICIREKVGEQAQVVIIDMNDPSNPIRRPISADSAIMNPASKVIALKAGKTLQIFNIEMKSKMKAHTMTDDVTFWKWISLNTVALVTDNAVYHWSMEGESQPVKMFDRHSSLAGCQIINYRTDAKQKWLLLTGISAQQNRVVGAMQLYSVDRKVSQPIEGHAASFAQFKMEGNAEESTLFCFAVRGQAGGKLHIIEVGTPPTGNQPFPKKAVDVFFPPEAQNDFPVAMQISEKHDVVFLITKYGYIHLYDLETGTCIYMNRISGETIFVTAPHEATAGIIGVNRKGQVLSVCVEEENIIPYITNVLQNPDLALRMAVRNNLAGAEELFARKFNALFAQGNYSEAAKVAANAPKGILRTPDTIRRFQSVPAQPGQTSPLLQYFGILLDQGQLNKYESLELCRPVLQQGRKQLLEKWLKEDKLECSEELGDLVKSVDPTLALSVYLRANVPNKVIQCFAETGQVQKIVLYAKKVGYTPDWIFLLRNVMRISPDQGQQFAQMLVQDEEPLADITQIVDVFMEYNLIQQCTAFLLDALKNNRPSEGPLQTRLLEMNLMHAPQVADAILGNQMFTHYDRAHIAQLCEKAGLLQRALEHFTDLYDIKRAVVHTHLLNPEWLVNYFGSLSVEDSLECLRAMLSANIRQNLQICVQVASKYHEQLSTQSLIELFESFKSFEGLFYFLGSIVNFSQDPDVHFKYIQAACKTGQIKEVERICRESNCYDPERVKNFLKEAKLTDQLPLIIVCDRFDFVHDLVLYLYRNNLQKYIEIYVQKVNPSRLPVVIGGLLDVDCSEDVIKNLILVVRGQFSTDELVAEVEKRNRLK...
example 2
n of Clathrin Light Chain
[0175]Clathrin light chain (below) was expressed in E. coli:
(SEQ ID NO: 4):MAELDPFGAPAGAPGGPALGNGVAGAGEEDPAAAFLAQQESEIAGIENDEAFAILDGGAPGPQPHGEPPGGPDAVDGVMNGEYYQESNGPTDSYAAISQVDRLQSEPESIRKWREEQMERLEALDANSRKQEAEWKEKAIKELEEWYARQDEQLQKTKANNRVADEAFYKQPFADVIGYVTNINHPCYSLEQAAEEAFVNDIDESSPGTEWERVARLCDFNPKSSKQAKDVSRMRSVLISLKQAPLVH(SEQ ID NO: 5):ATGGCGGAACTGGACCCGTTCGGCGCTCCGGCAGGCGCACCGGGCGGTCCGGCGCTGGGTAACGGCGTTGCGGGTGCTGGTGAAGAAGACCCGGCAGCAGCGTTCCTGGCGCAGCAGGAATCTGAAATCGCAGGTATCGAAAACGATGAAGCGTTCGCGATCCTGGACGGTGGTGCTCCGGGTCCGCAGCCGCACGGTGAACCGCCGGGTGGTCCGGATGCGGTTGACGGTGTTATGAACGGCGAGTACTACCAGGAGTCTAACGGTCCGACCGATTCTTACGCGGCAATTAGCCAGGTTGATCGTCTGCAaTCCGAACCGGAATCTATCCGTAAATGGCGTGAGGAGCAGATGGAACGCCTGGAAGCTCTGGACGCGAACTCTCGCAAACAGGAGGCGGAATGGAAAGAAAAAGCGATCAAAGAGCTGGAAGAATGGTATGCGCGTCAGGACGAACAGCTGCAaAAAACCAAAGCGAACAACCGTGTGGCGGACGAAGCATTCTACAAACAGCCGTTTGCGGACGTTATCGGTTACGTTACCAACATCAACCATCCGTGCTACTCTCTGGAGCAGGCAGCGGAAGAAGCgTTCGTGAACGACATCGACGAATCTAGCCCaGGcACCGAATGGG...
example 3
f Self-Assembled Protein
[0188]The self-assembled protein was loaded with a fluorescent compound to assess its ability to self-assembling following loading.
[0189]Recombinant clathrin heavy chain (HC) and light chain (LC) were diluted at 300 μg / mL and 800 μL / mL, respectively in 10 mM Tris-HCl (pH 7.9). A fluoresceinated test compound (FTC) was diluted at 500 μg / mL in the same buffer. Assembly of 100 μL in a 96-well assay plate was initiated by adding 4 μL of 1 M 2-(N-morpholino)ethanesulfonic acid (MES) buffer, pH 6.5 supplemented with 10 mM ethylene glycol tetraacetic acid (EGTA) and 75 mM CaCl2. A control was used with pH 7 MES buffer. OD320 nm readings were measured using the SpectraMax M3 (molecular devices) and the results were plotted by the software provided by the equipment.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com