Injectable Cryogel Vaccine Devices and Methods of Use Thereof
a cryogel and vaccine technology, applied in the direction of vertebrate antigen ingredients, prosthesis, antibody medical ingredients, etc., can solve the problems of high mortality rate, limited efficacy and safety options, and devastating cancers, and achieve the effect of increasing the efficacy of vaccine therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
e Biodegradable Preformed Macroscopic Geometric Gels
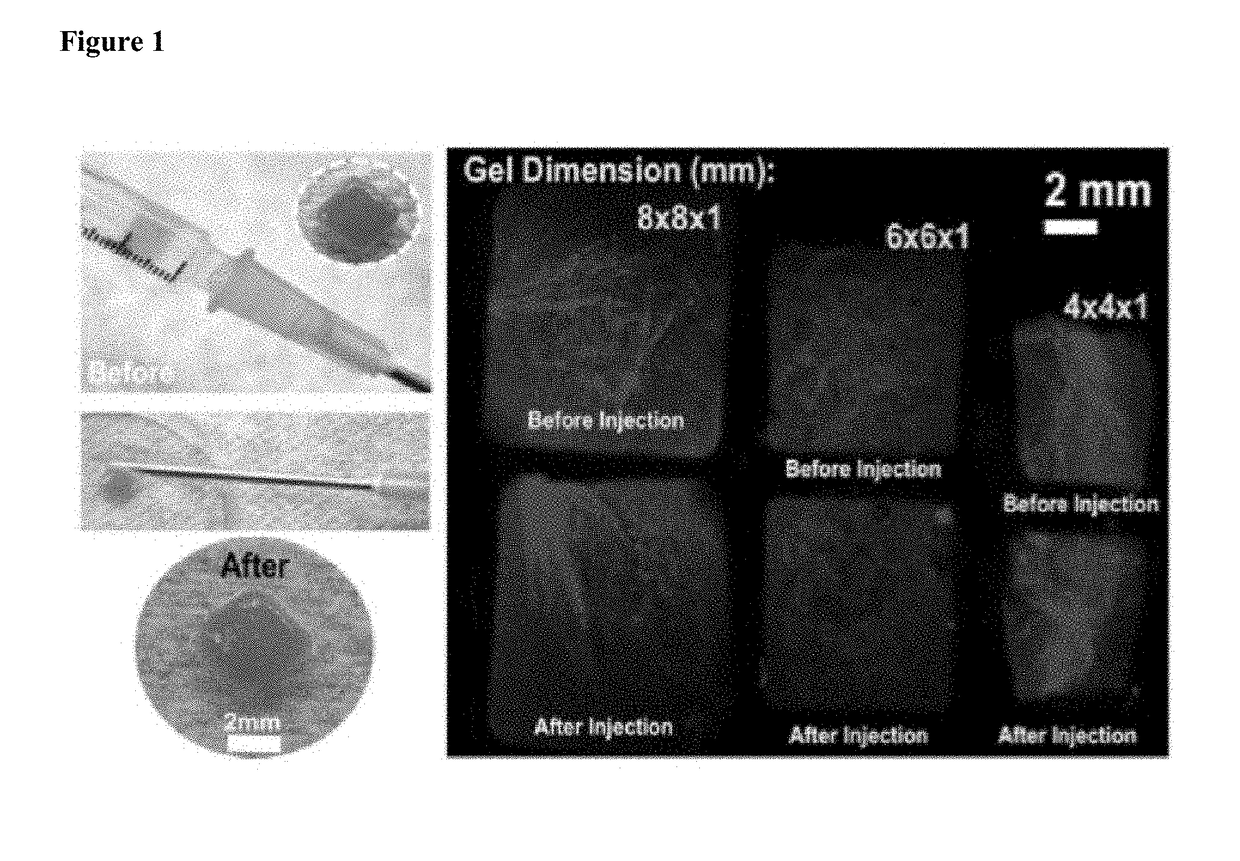
[0204]The compositions and methods described herein provide hydrogels for minimally invasive delivery of shape memory scaffolds for in vivo applications. This method has demonstrated highly efficient and reproducible fabrication of injectable shape-defined macroporous scaffolds. Although only one type of covalently alginate-based crosslinked gel system was evaluated herein, the material performance is readily manipulated by altering its composition, formulation, and degradation profile. The formation of specific shapes and structural stability are desirable characteristics for shape-defined materials, and the most important requirement of these types of materials for minimally invasive therapies is the ability to collapse and faithfully reform the scaffold's structure in a stimulus-responsive manner. A combination of mechanical compression and dehydration is sufficient to compress the scaffolds developed in this work, allowing mini...
example 2
l Integrity of Injectable Macroscopic Shape-Defined Gels
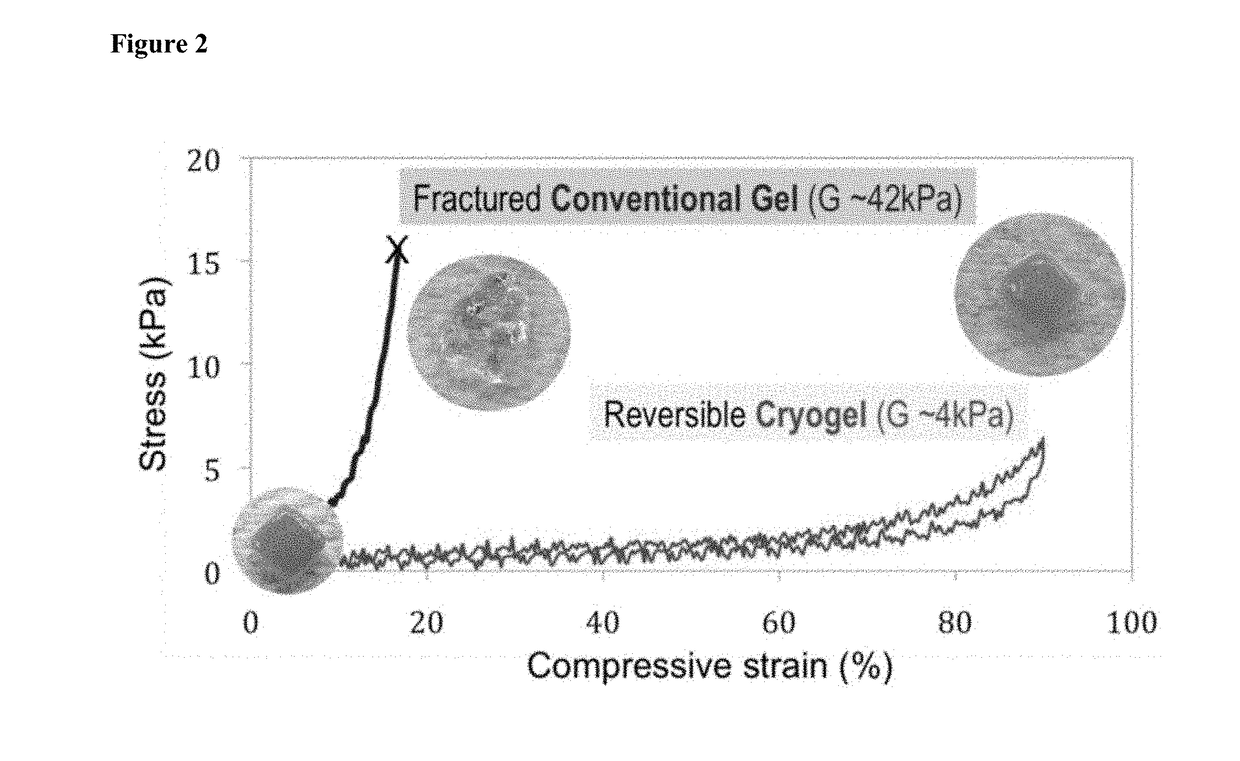
[0206]The deformation of conventional (nanoporous) and macroporous 1% MA-alginate gels under mechanical compression associated with shear forces was examined. Subject to mechanical compression, the gels experience a body of force, which results in a shape change. The influence of the macropores on the gel mechanical properties was also evaluated since the stiffness of the scaffold dictate the extent of the deformation under an applied shear force. Conventional gels give a Young's modulus (i.e., the slope of the initial part of the stress vs. strain curves in FIG. 2) of 42±4 kPa in compression test. However, macroporous gels led to a dramatic reduction in the modulus to 4±2 kPa. As shown in FIG. 2, cylindrical (4 mm diameter×8 mm height) nanoporous gels reduced their heights by ˜16% when subjected to a vertical load before mechanical fracture. In comparison, cylindrical macroporous gels give much larger deformation under lower m...
example 3
ory Injectable Scaffolds As a Controlled Drug Delivery Carrier
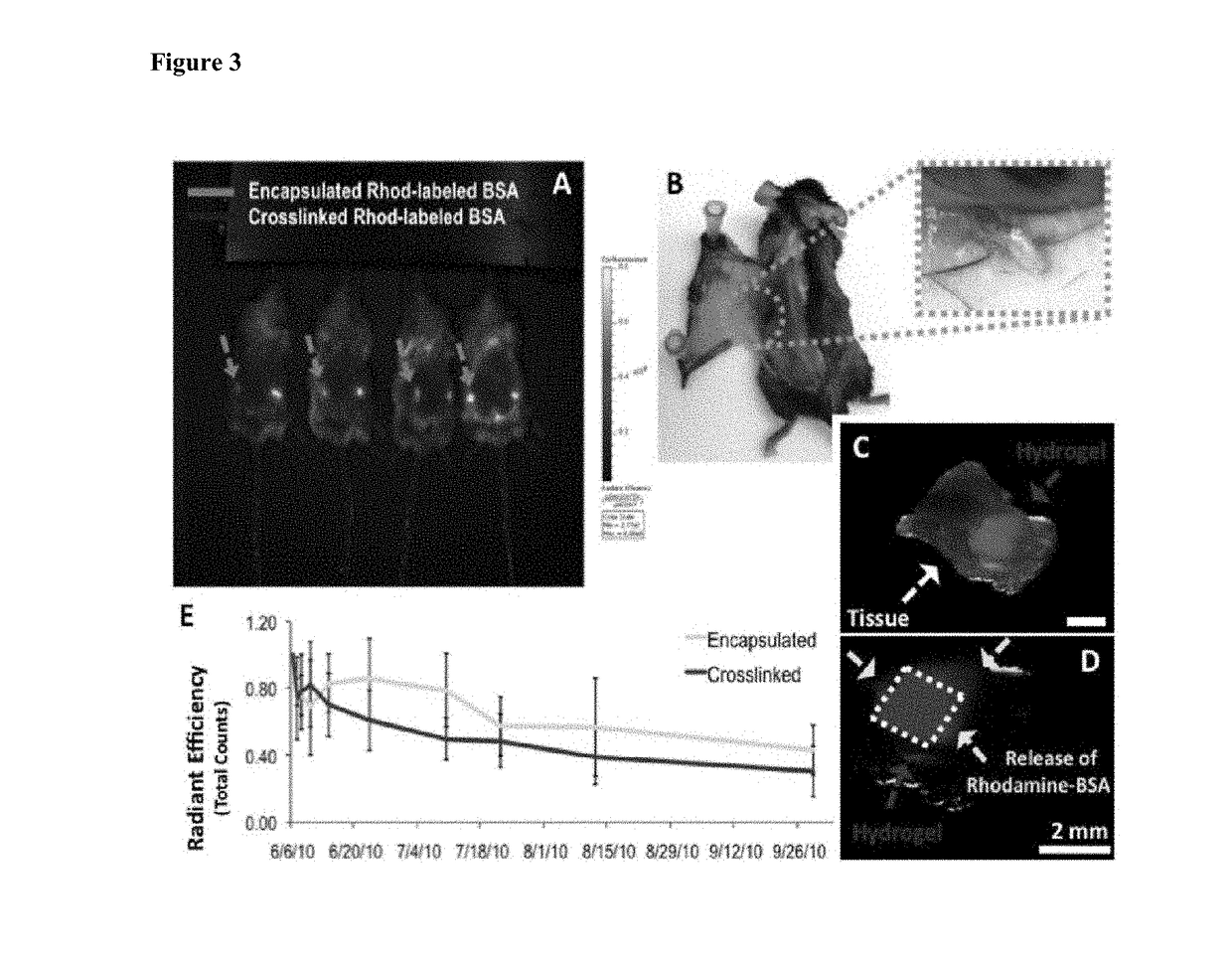
[0208]Covalently crosslinked alginate scaffolds possessing shape memory properties were successfully used as a drug delivery system in vivo. The gels having a predefined size and structure were able to exceptionally maintain their structural features after minimally invasive subcutaneously insertion in mice. Suspended gels in PBS were spontaneously hydrated with full geometric restoration after one single injection per site on the lower back of mice. Injected animals did not demonstrate abnormalities in feeding, grooming, or behavior during the time frame of the experiment, nor did they exhibit signs of distress.
[0209]The hydrogels maintained their hydrogel shape integrity at the site of injection. Animal studies performed to examine the integration of the spongy-like gels with the host tissue showed that the alginate-based scaffolds were biocompatible and did not elicit an immune response or rejection when injected in mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com