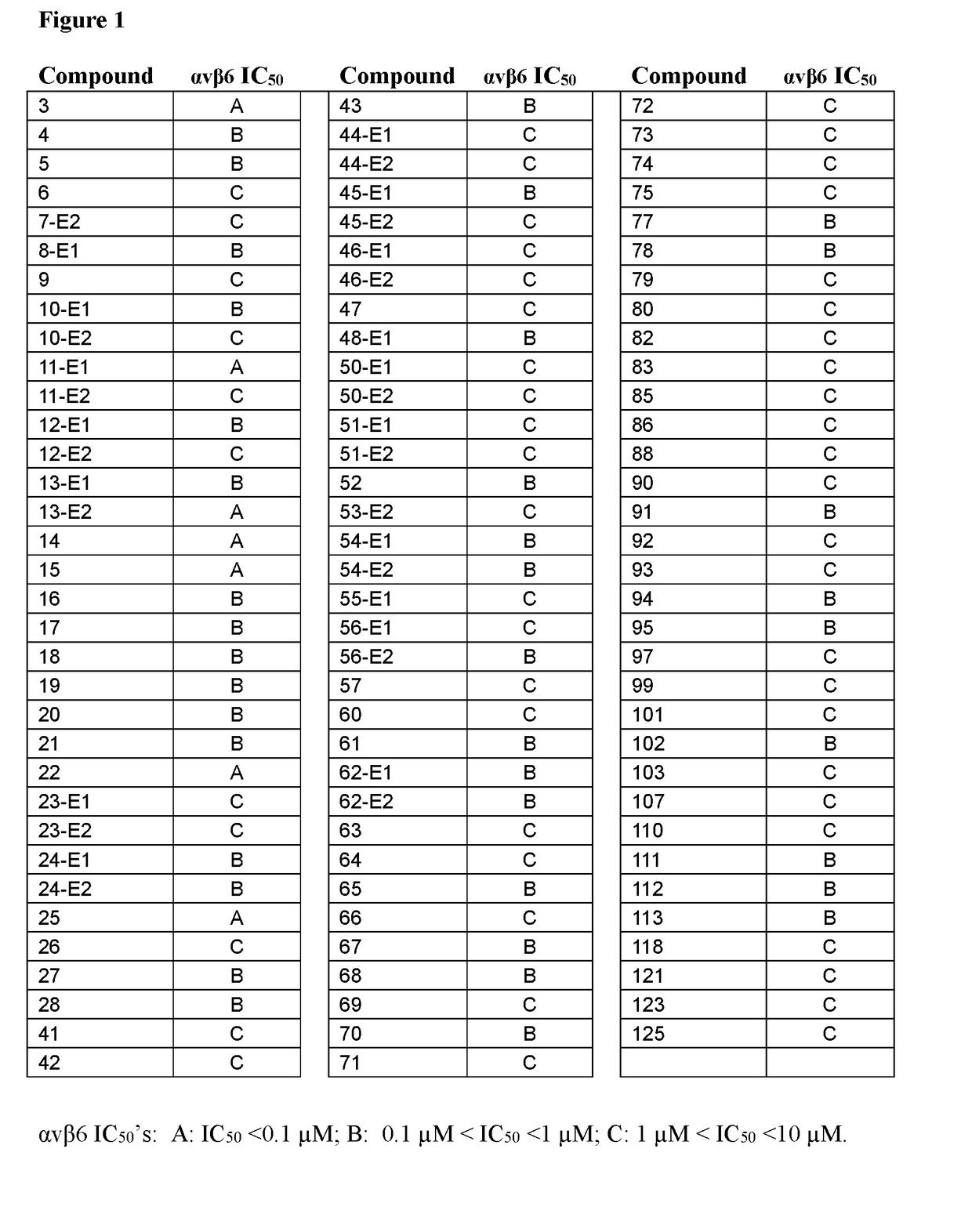
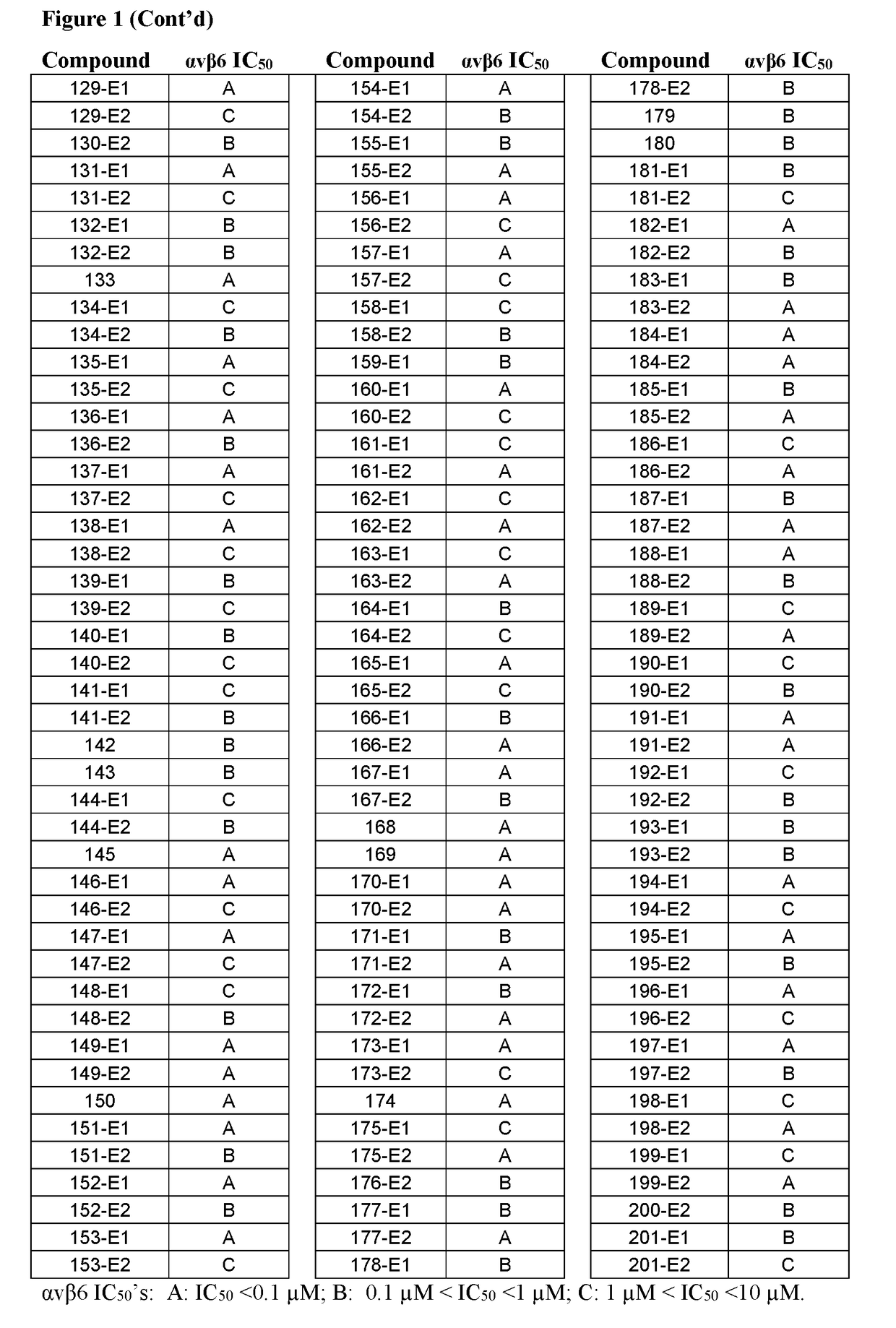
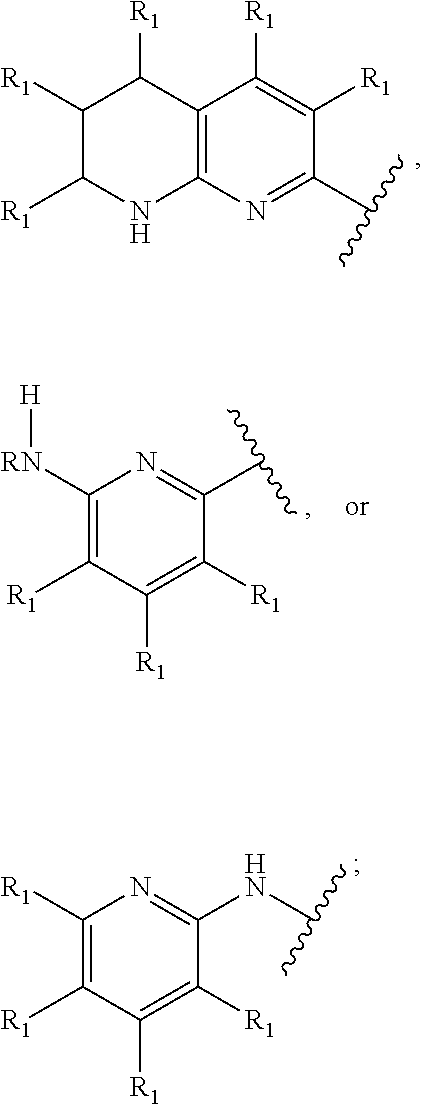
Inhibitors of (alpha-v)(beta-6) integrin
a technology of beta-v and beta-v, which is applied in the field of inhibitors of beta-v (beta-6) integrin, can solve the problems of not being able to achieve therapeutic success with orally bioavailable integrin inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
on of 2-(4-((4-(5,6,7,8-tetrahydro-1,8-naphthyridin-2-yl)piperidin-1-yl)methyl)piperidin-1-yl)acetic acid (compound 1)
Step 1: tert-butyl 4-(1,8-naphthyridin-2-yl)piperidine-1-carboxylate
[0185]
[0186]A mixture of tert-butyl 4-acetylpiperidine-1-carboxylate (2.0 g, 8.80 mmol), 2-aminonicotinaldehyde (1.1 g, 8.80 mmol) and L-proline (2.0 g, 17.60 mmol) in EtOH (20 mL) was heated to reflux overnight. Solvent was removed in vacuo, and the residue was purified by silica gel column (pet ether:EtOAc=1:1) to give tert-butyl 4-(1,8-naphthyridin-2-yl)piperidine-1-carboxylate as a colorless oil (0.8 g). Yield 30% (100% purity, UV=214 nm, ESI 314.2 (M+H)+).
Step 2: tert-butyl 4-(5,6,7,8-tetrahydro-1,8-naphthyridin-2-yl)piperidine-1-carboxylate
[0187]
[0188]Tert-butyl 4-(1,8-naphthyridin-2-yl)piperidine-1-carboxylate (0.8 g, 2.56 mmol) was hydrogenated over Pd—C (100 mg, 10% on activated carbon) under balloon hydrogen in EtOH (20 mL) at room temperature overnight. The reaction was filtered through Ce...
example 2
on of 2-(2-oxo-4-(4-((5,6,7,8-tetrahydro-1,8-naphthyridin-2-yl)methyl) piperidine-1-carbonyl)piperazin-1-yl)acetic acid (compound 2)
Step 1: tert-butyl 4-(2-methoxy-2-oxoethyl)-3-oxopiperazine-1-carboxylate
[0199]
[0200]To a solution of tert-butyl 3-oxopiperazine-1-carboxylate (5.00 g, 25.0 mmol) in DMF (50 mL) at 0° C. was added NaH (60% in mineral oil, 1.20 g, 30.0 mmol). The mixture was stirred for 30 min, and then methyl 2-bromoacetate (2.60 mL, 27.5 mmol) was added. The reaction was stirred at room temperate for 16 hours, then quenched with H2O (50 mL) and extracted with EtOAc (50 mL×3). The combined organic extracts were washed with brine, dried over Na2SO4, filtered and concentrated in vacuo. The residue was purified by silica gel column (pet ether:EtOAc 5:1 to 2:1) to afford the desired compound as a colorless oil (4.0 g). Yield 59% (86% purity, UV=214 nm, ESI 217.0 (M+H)+).
Step 2: methyl 2-(2-oxopiperazin-1-yl)acetate
[0201]
[0202]Tert-butyl 4-(2-methoxy-2-oxoethyl)-3-oxopiperaz...
example 3
on of 2-(2-oxo-4-(4-(5,6,7,8-tetrahydro-1,8-naphthyridin-2-yl)piperidine-1-carbonyl)piperidin-1-yl)acetic acid (compound 3)
[0207]Step 1: methyl 1-(2-tert-butoxy-2-oxoethyl)-2-oxopiperidine-4-carboxylate
[0208]To a solution of methyl 2-oxopiperidine-4-carboxylate (2.90 g, 18.4 mmol) in DMF (30 mL) at 0° C. was added NaH (60% in mineral oil, 886 mg, 22.1 mmol). The mixture was stirred for 30 min, and then tert-butyl 2-bromoacetate (4.32 g, 22.1 mmol) was added. The reaction was stirred at room temperature for 16 hours, then quenched with water (20 mL), and extracted with EtOAc (50 mL×5). The combined organic extracts were washed with brine, dried over Na2SO4, filtered and concentrated in vacuo. The residue was purified by silica gel column (pet ether:EtOAc 4:1) to give methyl 1-(2-tert-butoxy-2-oxoethyl)-2-oxopiperidine-4-carboxylate as a colorless oil (1.5 g). Yield 30% (90% purity, UV=214 nm, ESI 216.1 (M+H)+).
Step 2: 1-(2-tert-butoxy-2-oxoethyl)-2-oxopiperidine-4-carboxylic acid
[020...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| detection wavelength | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com