Method for reproducible differentiation of clinical-grade retinal pigment epithelium cells
a retinal pigment epithelium and reproducible technology, applied in the field of stem cell biology, can solve the problems of degeneration of the retina, loss of visual function, blindness, lack of efficient large-scale production of ipsc- or esc-derived rpe cells, etc., and achieve the effect of varying efficiency and not being scalabl
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
on of Starting Pluripotent Stem Cell Population
[0176]A starting population of RPE cells can be derived from pluripotent stem cells such as ES cells and iPSCs. In exemplary methods, the RPE cells were derived from human iPSCs reprogrammed from somatic cells by methods known in the art such as U.S. Pat. Nos. 8,546,140, 8,741,648, 8,691,574, Published U.S. Patent Application No. 20090246875, Published U.S. Pat. No. 8,278,104, Published U.S. Pat. Nos. 9,005,967, 8,058,065, 8,129,187, PCT Publication NO. WO 2007 / 069666 A1, U.S. Pat. Nos. 8,183,038 and 8,268,620, which are incorporated herein by reference. In one exemplary method, nuclear programming factors Oct4, Sox2, c-Myc and Klf4 were used to produce pluripotent stem cells from a somatic cell. In another exemplary method, nuclear programming factors Oct4, Sox2, Nanog, Lin28, L-Myc, and SV40 Large T-antigen were used to produce pluripotent stem cells from a somatic cell.
[0177]The iPSCs were grown without mouse or human feeder layers i...
example 2
iation of iPSCs into RPE Cells
[0180]Once the single cell iPSCs seeded at the appropriate cells density were cultured for about 2 days as in Example 1, they were cultured in various differentiation media for deriving RPE cells. On day 3, the E8™ medium was aspirated and room temperature Retinal Induction Medium (RIM) (e.g., Table 3) was added. Briefly, the RIM comprised DMEM and F12 at about a 1:1 ratio, knockout serum replacement, MEM non-essential amino acids (NEAA), sodium pyruvate, N-2 supplement, B-27 supplement, and ascorbic acid. In addition, the RIM comprised a WNT pathway inhibitor, a BMP pathway inhibitor, a TGFβ pathway inhibitor and insulin growth factor 1 (IGF1). Each day the media was aspirated and fresh RIM was added to the cells. The cells were cultured in the RIM for about two to four days.
[0181]The cells were next cultured in Retinal Differentiation Medium (RDM) for about seven to fourteen days. Briefly, the RDM (Table 2) comprised DMEM and F12 at about a 1:1 ratio,...
example 3
n of RPE Cells
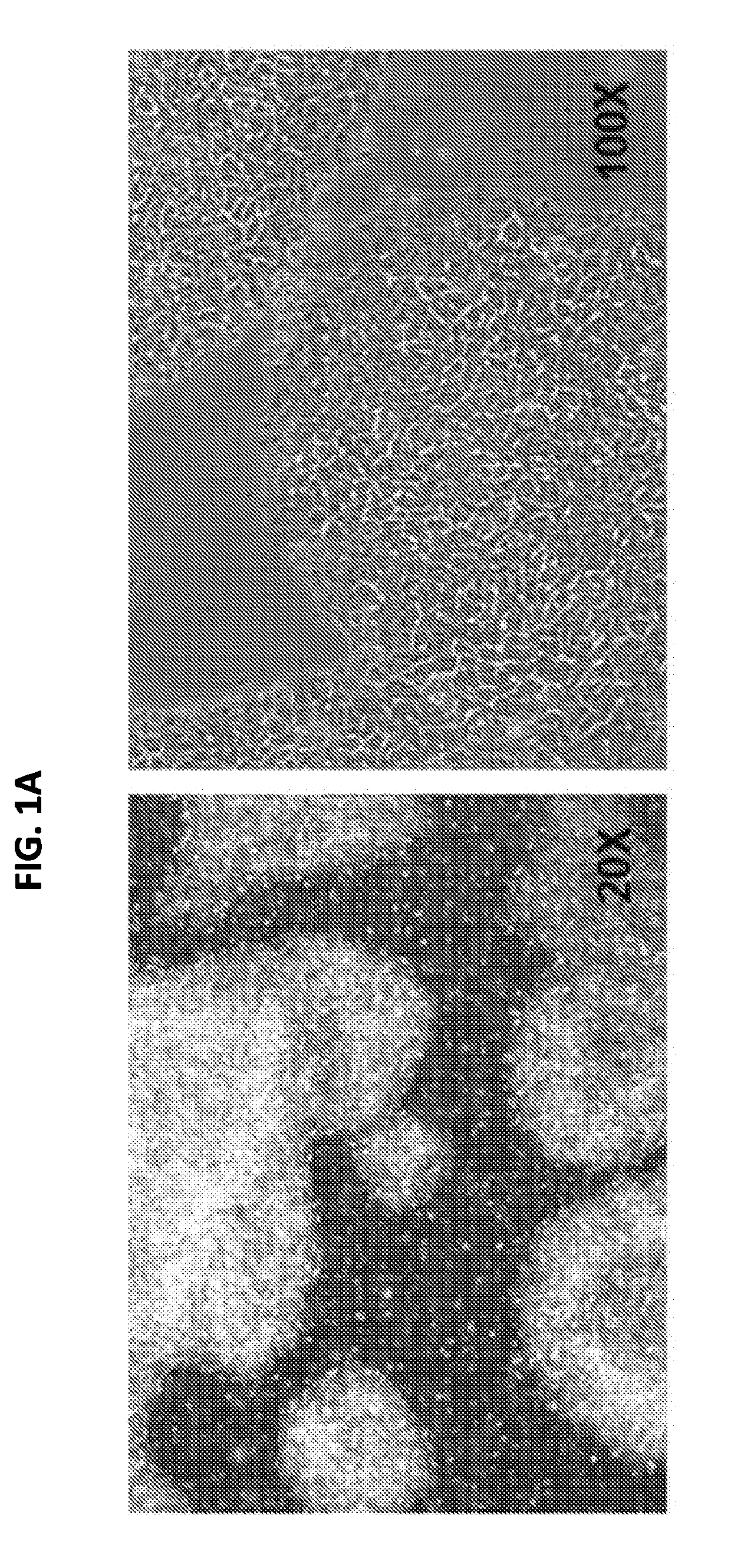
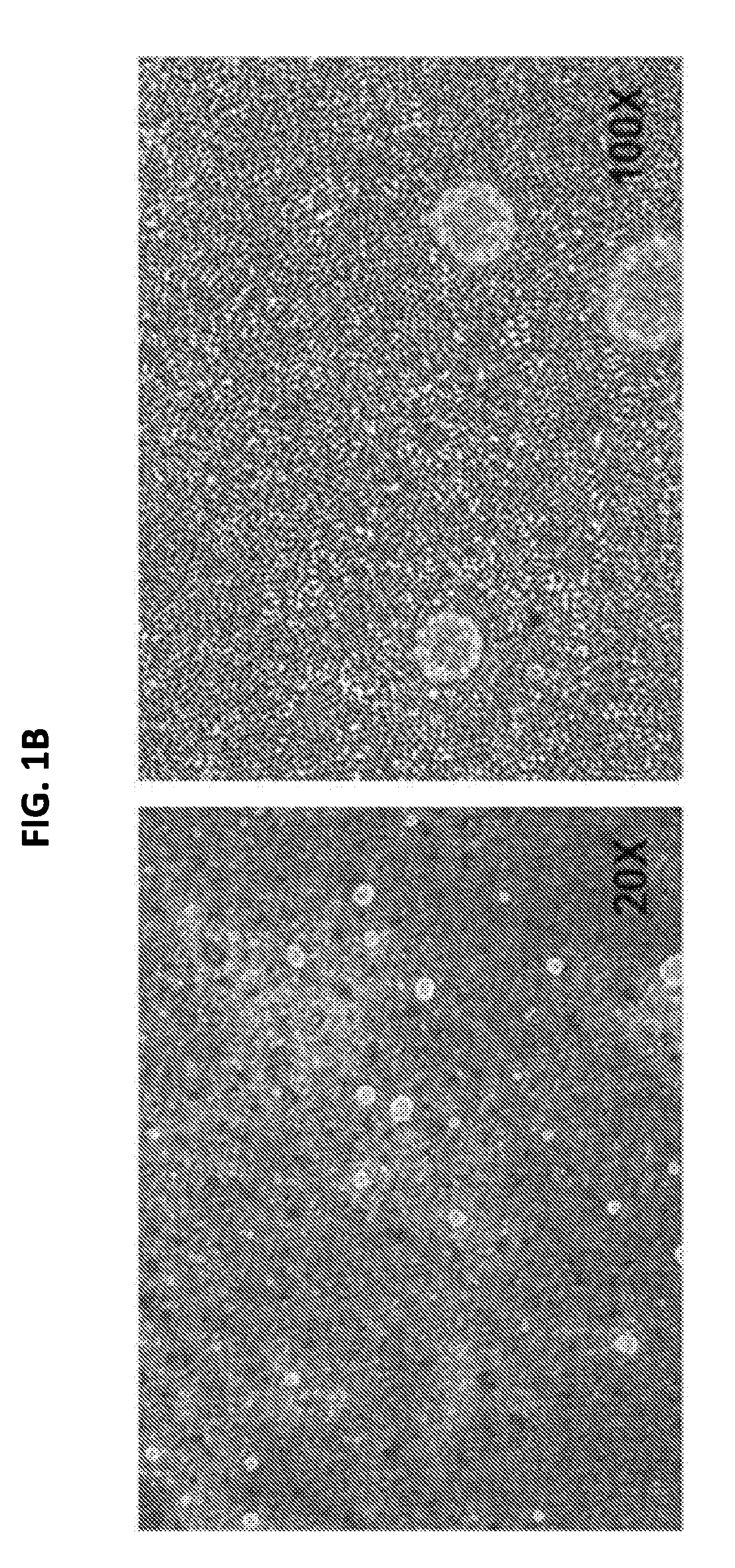
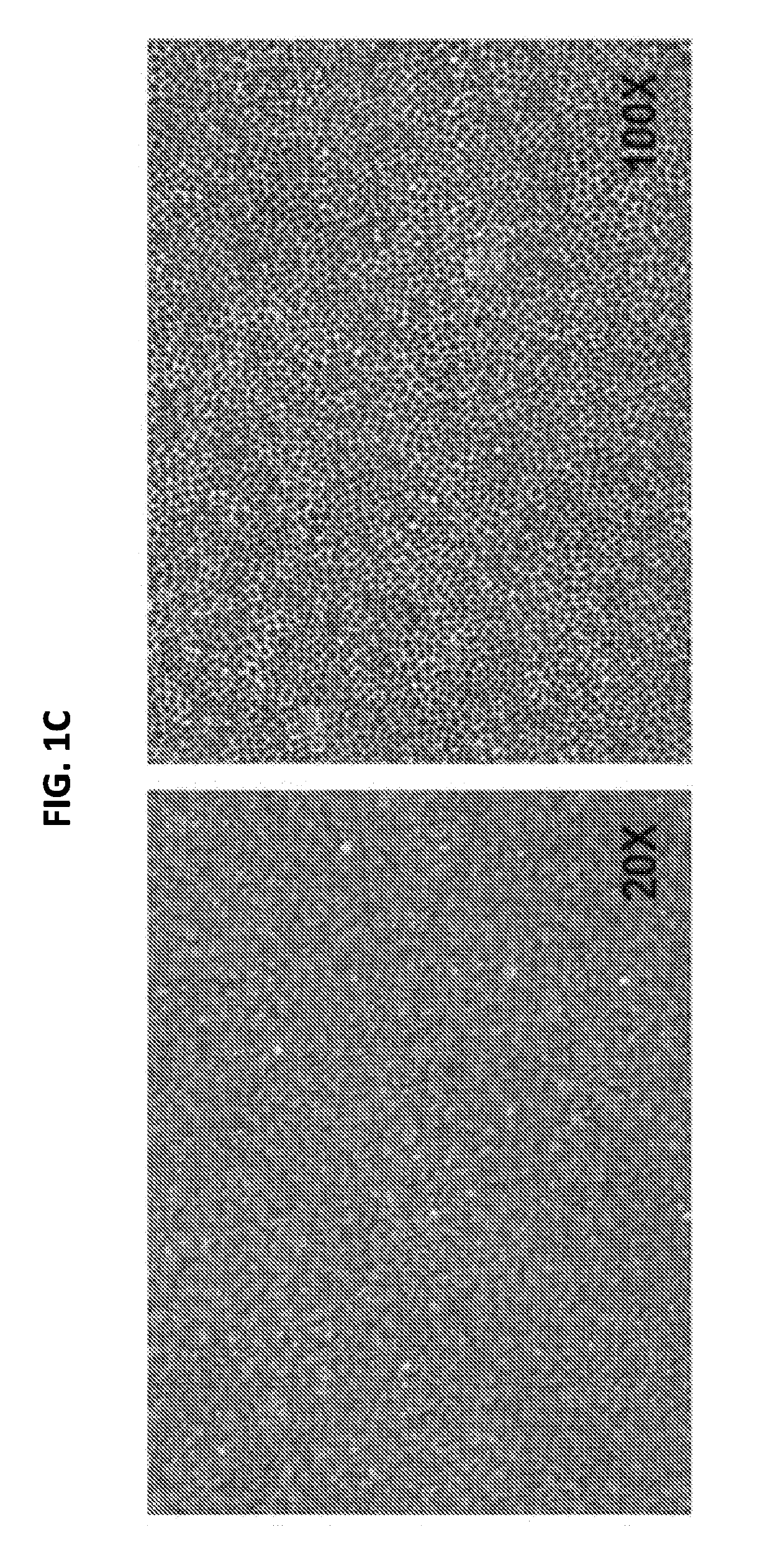
[0184]For continued maturation of the RPE cells produced in Example 2, the cells were dissociated in a cell dissociation enzyme such as TRYPLE™ and reseeded on a degradable scaffold assembly in a specialized SNAPWELL™ design for 1-2 weeks in the RPE-MM with a MEK inhibitor such as PD325901. This resulted in differentiated, polarized, and confluent monolayers of functional RPE cells (FIG. 1D) which can be cryopreserved at this stage in xenofree CS10 medium.
[0185]The mature RPE cells were further developed into functional RPE cell monolayers that function as an intact RPE tissue by continued culture in the RPE-MM with additional small molecules such as primary cilium inducers like PGE2 or aphidicolin. Without being bound by theory, these primary cilium inducers suppress the canonical WNT pathway, induce cell cycle exit in the cells, and induce apical-basal polarization in the RPE monolayer. RPE maturity can alternatively be induced by canonical WNT pathway inhibitors suc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com