Transdermal Therapeutic System for 5-Aminolevulinic Acid Hydrochloride
a technology of aminolevulinic acid and transdermal therapy, which is applied in the direction of dermatological disorders, drug compositions, peptide/protein ingredients, etc., can solve the problems of only comparatively poor penetration of aminolevulinic acid through human skin, and the death of corresponding cells, etc., to achieve adequate stickyness, easy to apply and remove, and good tolerated by skin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0043]The transdermal therapeutic system produced contains the following constituents:
%(% by weight / % by weight)mg / PlasterActive ingredient:5-aminolevulinic19.710.2acid hydrochloridePolymera):DURO-TAK49.625.8387-2353Back layer:3M SCOTCHPAK ™30.716.01109Protective layer:polyethyleneb)59.0c)terephthalate layersiliconised on oneside (75 μm)a)in ethyl acetate and hexane, which are both virtually completely removed during the drying processb)is removed before applicationc)estimated
[0044]In relation to the polymer matrix, 28% by weight 5-aminolevulinic acid hydrochloride and 72% by weight DURO-TAK 387-2353 (polyacrylate without cross-linking agent) are accordingly present.
example 2
[0045]The composition of this example corresponds to Example 1, except that instead of DURO-TAK 387-2353, the same quantity of DURO-TAK 387-2052 (polyacrylate with cross-linking agent) was used.
example 4
[0047]The release rate was measured using the so-called “paddle over disc” method, as described in European Pharmacopoeia 6.0, 2.9.4. “dissolution test for transdermal patches”, January 2008: 20904, under the following conditions:
Apparatus used: paddle over disc
Release medium: citrate buffer pH 3.0
Volume of the release medium: 300 ml
Temperature: 32° C.±0.5° C.
[0048]Rotation frequency: 50 min−1
Sample removal time: 0.5 h, 2 h and 7 h
Sample volume: 10.0 ml
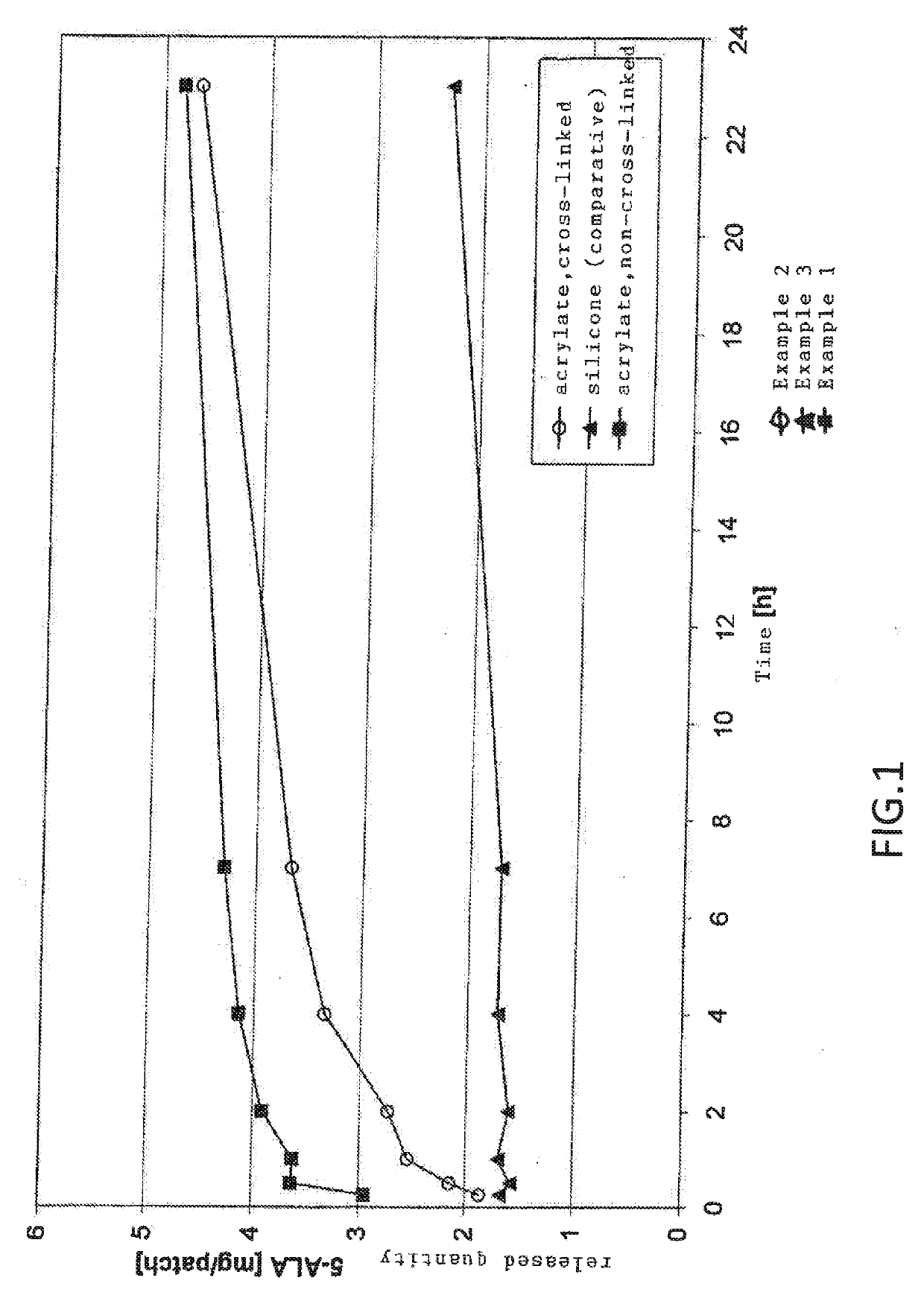
[0049]The results are shown in FIG. 1. Specifically, FIG. 1 is a graph showing released quantity 5-ALA [mg / patch] versus time [h] for Examples 1, 2, and 3. Example 1 is acrylate (non cross-linked), Example 2 is acrylate (cross-linked), and Example 3 is silicone (comparative).
[0050]The release rate of a transdermal therapeutic system according to Example 1 is higher than that according to Example 2. Both Example 1 and Example 2 exhibit a clearly faster release compared to comparative Example 3. Furthermore, the stability of the 5-amin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com