Liver organoid compositions and methods of making and using same
a technology of liver organs and compositions, applied in the field of liver organoid compositions and methods of making and using same, can solve the problems of limiting the practical use of liver cells in the pharmaceutical industry, wasting billions of dollars annually in drug development, and the functionality of the existing methodology using liver cells is extremely poor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
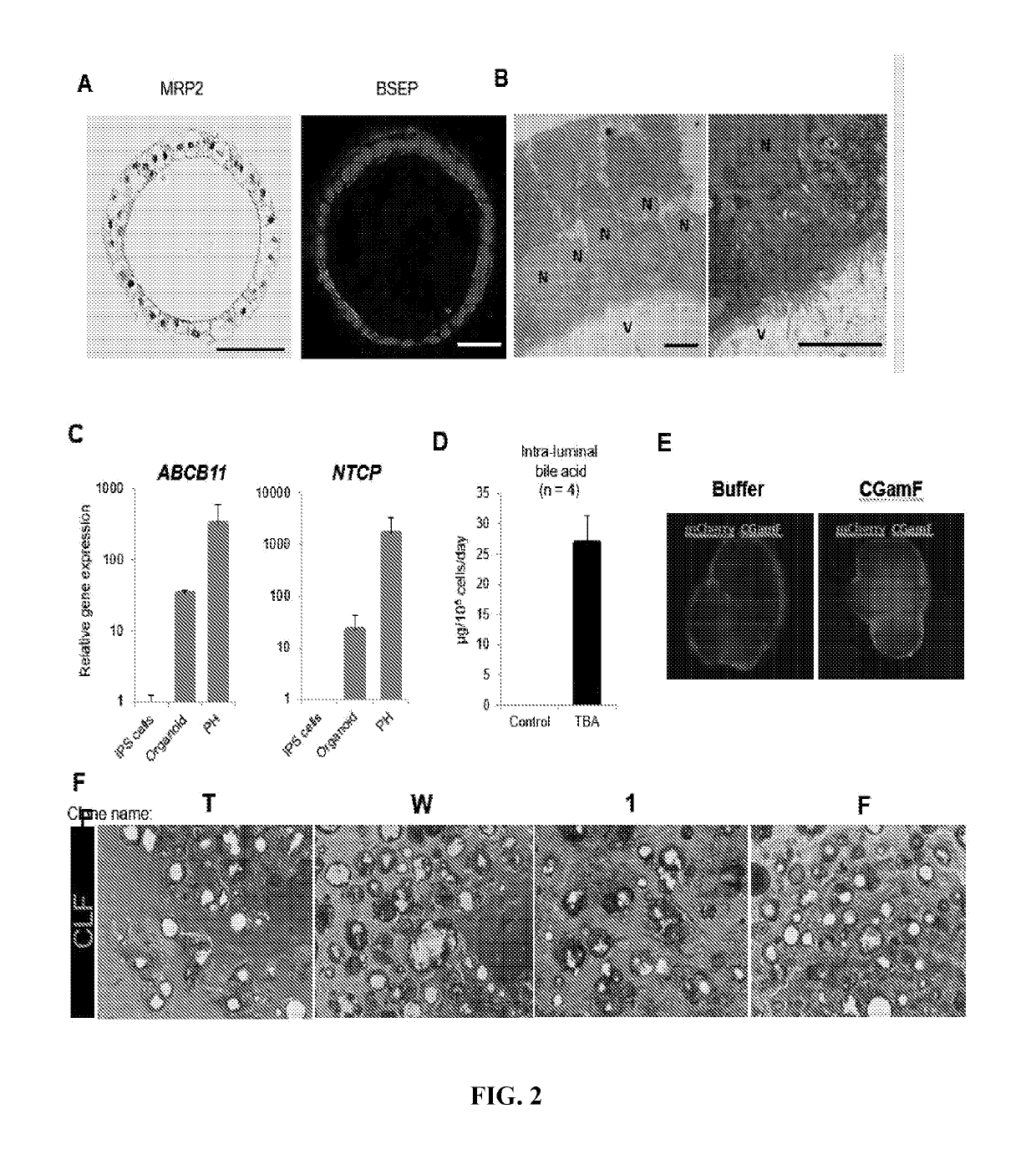
[0059]In the present study, Applicant tested bile transport activity using Fluorescein Diacetate, which was excreted by MRP2 across the canalicular membrane into the bile canalicular networks (Tian et al., 2004). It has previously been reported that Troglitazone and Cyclosporin inhibit the MRP2 (Chang et al., 2013; Lechner et al., 2010). In addition, the efflux transporter MRP2 mediates export of Bosentan (Fahrmayr et al., 2013). Although the inhibition of MRP2 by Nefazodone was not reported, mitochondria stress by Nefazodone may be related to a decrease of the bile transport activity, efflux of Fluorescein Diacetate, because MRP2 is an ATP-dependent bile salt transporter for canalicular excretion of bile acids in hepatocytes.
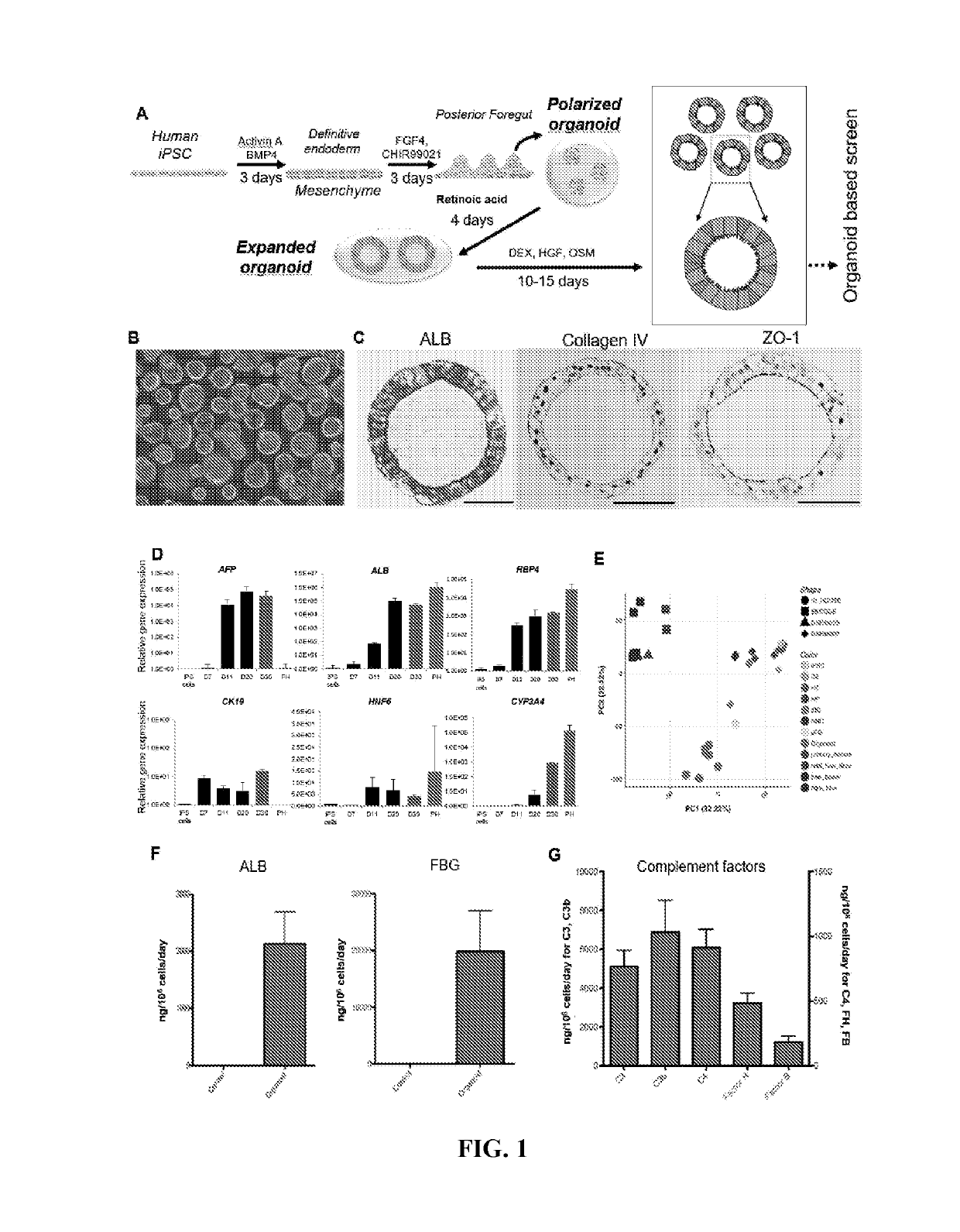
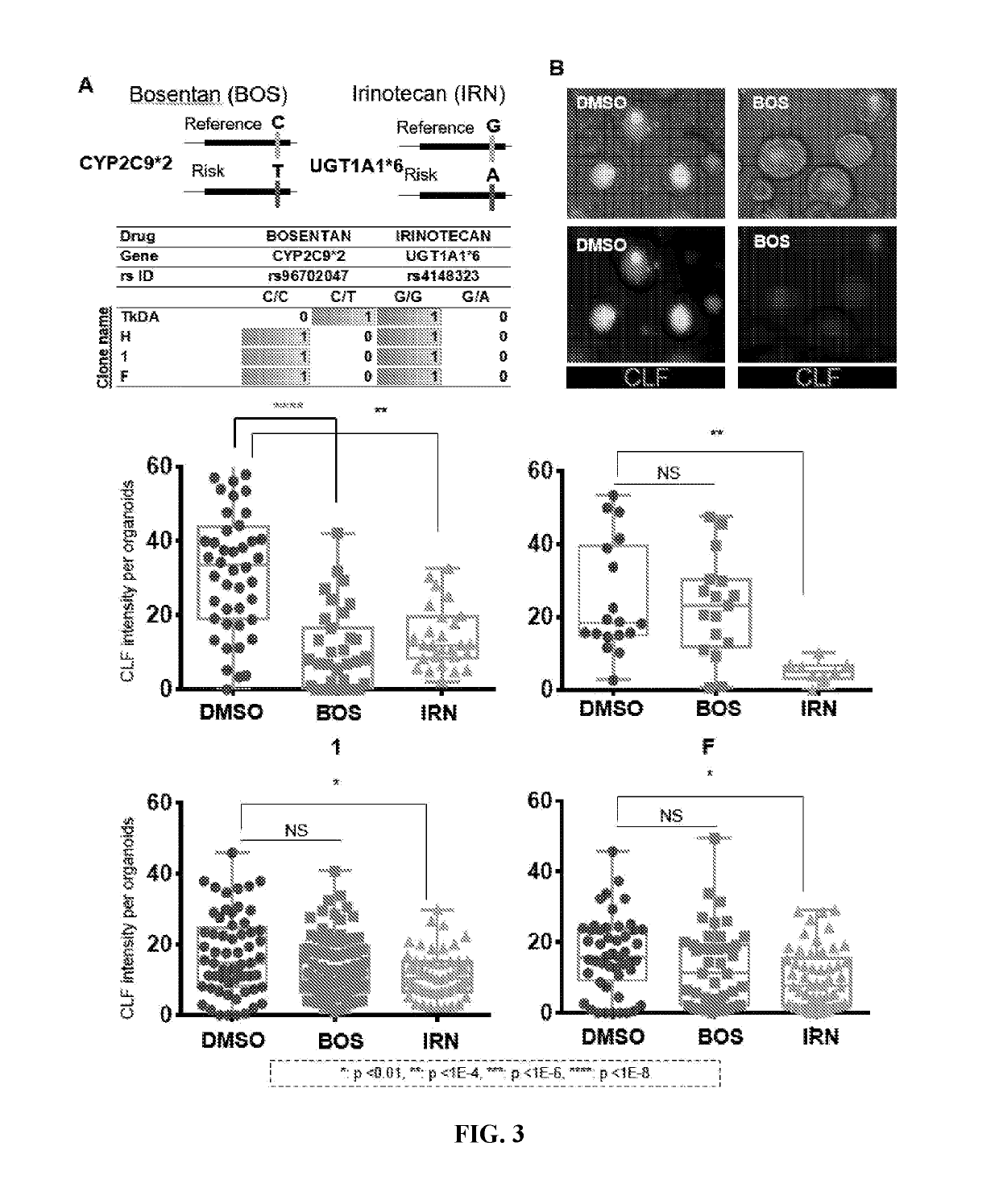
[0060]Preclinical detection of risk compounds for drug induced liver injury (DILI) remains a significant challenge in drug development, highlighting a need for a predictive human system. Here, Applicant developed a human liver organoid (HLO) model for analyzing...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com