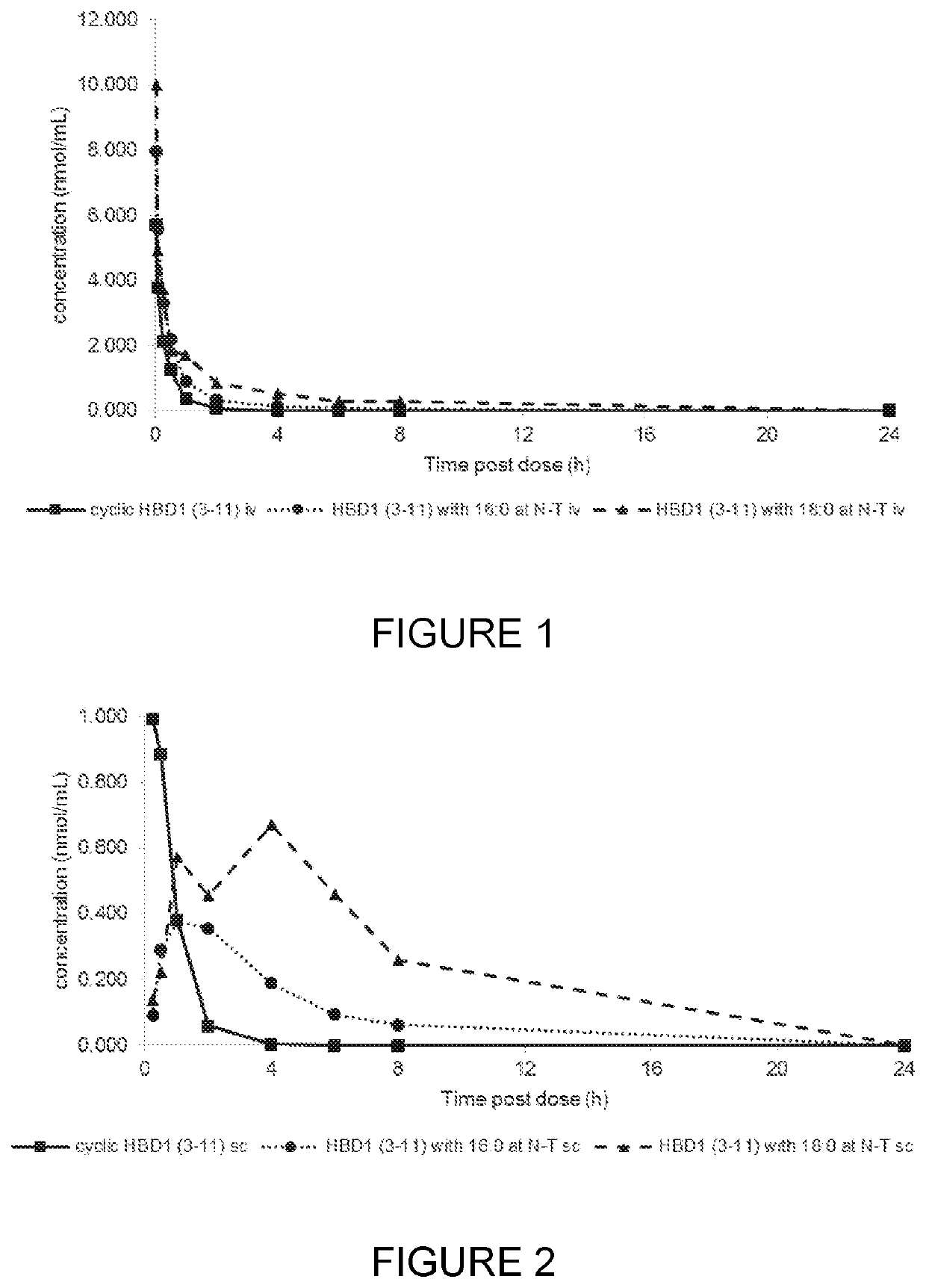
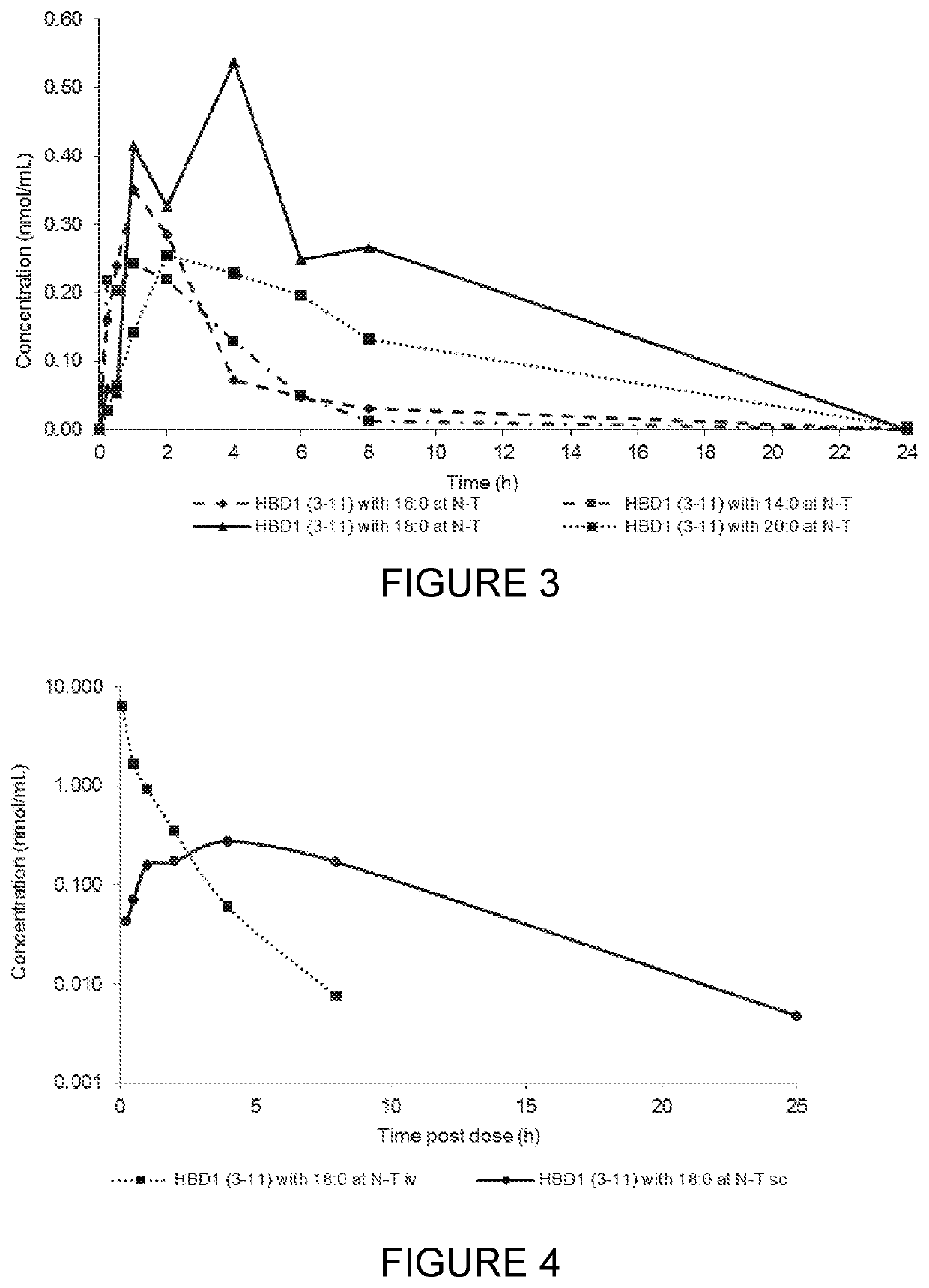
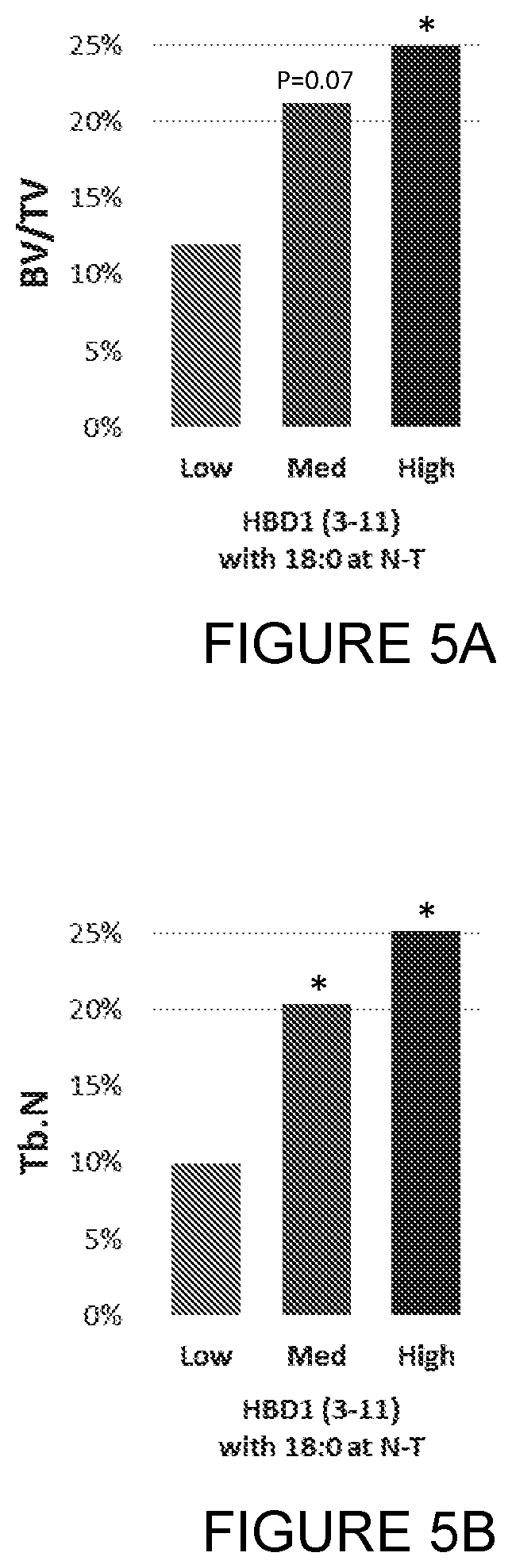
Compounds, compositions and uses thereof for improvement of bone disorders
a technology of compounds and compounds, applied in the field of compounds, can solve the problems of long peptides being dangerous for patients, long peptides are usually rapidly degraded, and the manufacturing process is extensive and expensive, so as to prevent or treat bone disorders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
HBD1 Fragments on Osteocalcin Expression During In Vitro Osteoblast Differentiation
[0124]Peptides were manufactured according to a standard manufacturing process in peptide chemistry by solid phase peptide synthesis (SPPS) using the Fmoc (9-fluorenylmethyloxycarbonyl) strategy (Merrifield, R. B. Solid phase peptide synthesis. I. The synthesis of a tetrapeptide. J. Am. Chem. Soc. 1963, 85, 2149-2154). Identity of the peptides was verified by LC-MS. The purity (at least 95%) and the net peptide content of peptides were determined by RP-HPLC and elemental analysis, respectively.
[0125]Each peptide was tested in a biologic assay measuring its ability to stimulate differentiation of osteoblast cells over a 18-21 day interval (Table 8) or a 14-15 day interval (Table 9) as assessed by the stimulation of osteocalcin protein synthesis. MC-3T3 E1 clone 4 (CL4) osteoblast cells were obtained from ATCC (Manassas, Va., USA). Cells were cultured in α-MEM containing 10% fetal bovine serum (FBS; The...
example 2
f Various Doses of HBD1 Fragments on Osteocalcin Expression During In Vitro Osteoblast Differentiation
[0127]The HBD1 (3-11) peptide as set forth in SEQ ID NO: 10 was manufactured according to a standard manufacturing process in peptide chemistry by solid phase peptide synthesis (SPPS) using the Fmoc (9-fluorenylmethyloxycarbonyl) strategy (Merrifield, R. B. Solid phase peptide synthesis. I. The synthesis of a tetrapeptide. J. Am. Chem. Soc. 1963, 85, 2149-2154). The identity of the peptide was verified by LC-MS. The purity (at least 95%) and the net peptide were determined by RP-HPLC and elemental analysis, respectively.
[0128]The peptide was tested at different concentrations in a biologic assay measuring its ability to stimulate differentiation of osteoblast cells over a 18 day interval as assessed by the stimulation of osteocalcin protein synthesis. MC-3T3 E1 clone 4 (CL4) osteoblast cells were obtained from ATCC (Manassas, Va., USA). Cells were cultured in α-MEM containing 10% fe...
example 3
HBD1 Fragment Analogs with Alanine Substitutions on Osteocalcin Expression During In Vitro Osteoblast Differentiation
[0130]The peptides were manufactured according to a standard manufacturing process in peptide chemistry by solid phase peptide synthesis (SPPS) using the Fmoc (9-fluorenylmethyloxycarbonyl) strategy (Merrifield, R. B. Solid phase peptide synthesis. I. The synthesis of a tetrapeptide. J. Am. Chem. Soc. 1963, 85, 2149-2154). Identity of peptides was verified by LC-MS. The purity (at least 95%) and the net peptide content of peptides were determined by RP-HPLC and elemental analysis, respectively.
[0131]Each peptide was tested in a biologic assay measuring its ability to stimulate differentiation of osteoblast cells over a 15 day interval as assessed by the stimulation of osteocalcin protein synthesis. MC-3T3 E1 clone 4 (CL4) osteoblast cells were obtained from ATCC (Manassas, Va., USA). Cells were cultured in α-MEM containing 10% fetal bovine serum (FBS; Thermo Fisher Sc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com