Targeted oligonucleotides
a technology of oligonucleotides and oligonucleotides, which is applied in the field of aptamers, can solve the problems of scalability and cost, severe limitations, and extremely difficult to elicit antibodies to aptamers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
ation of DNA Oligonucleotides that Bind a Target
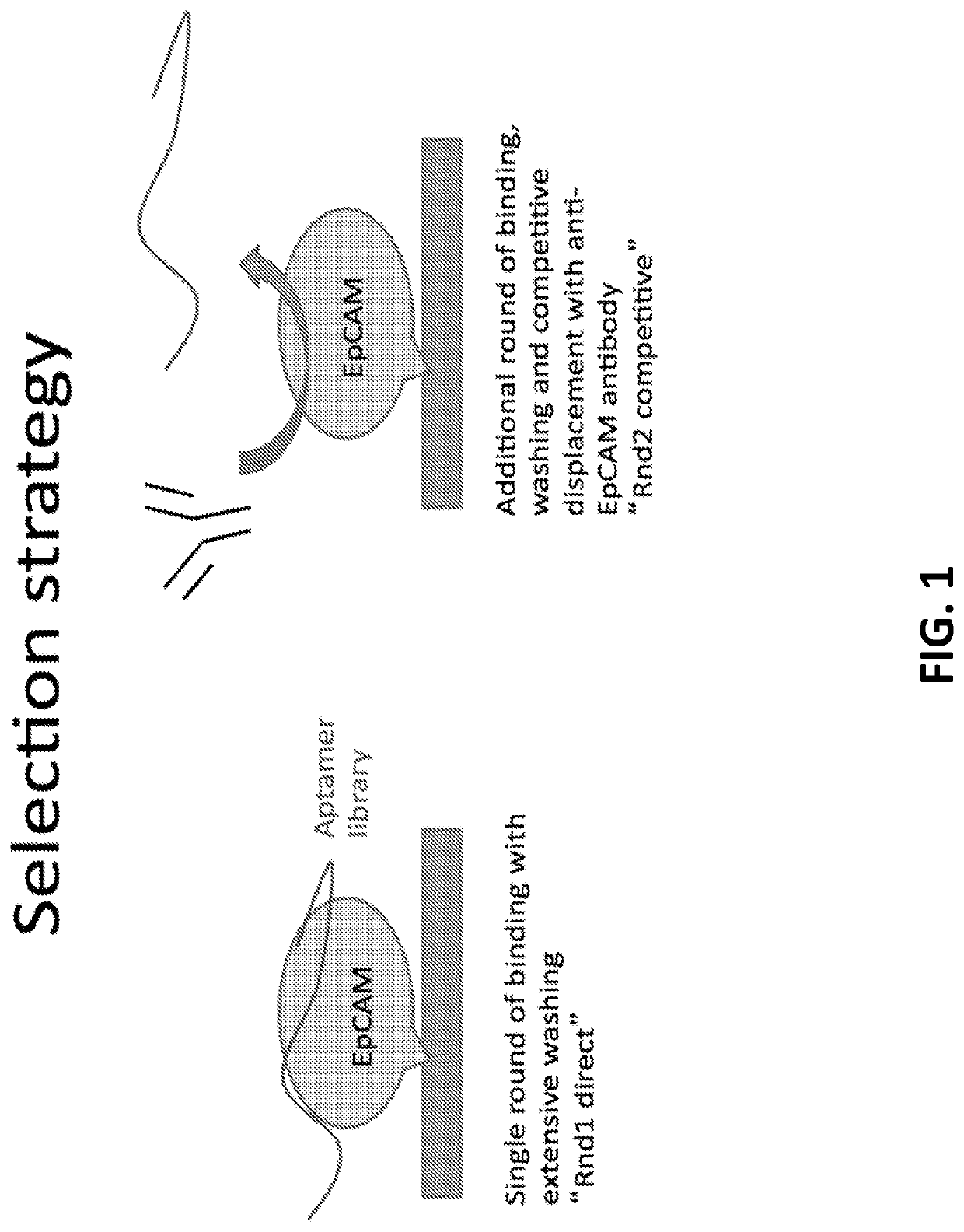
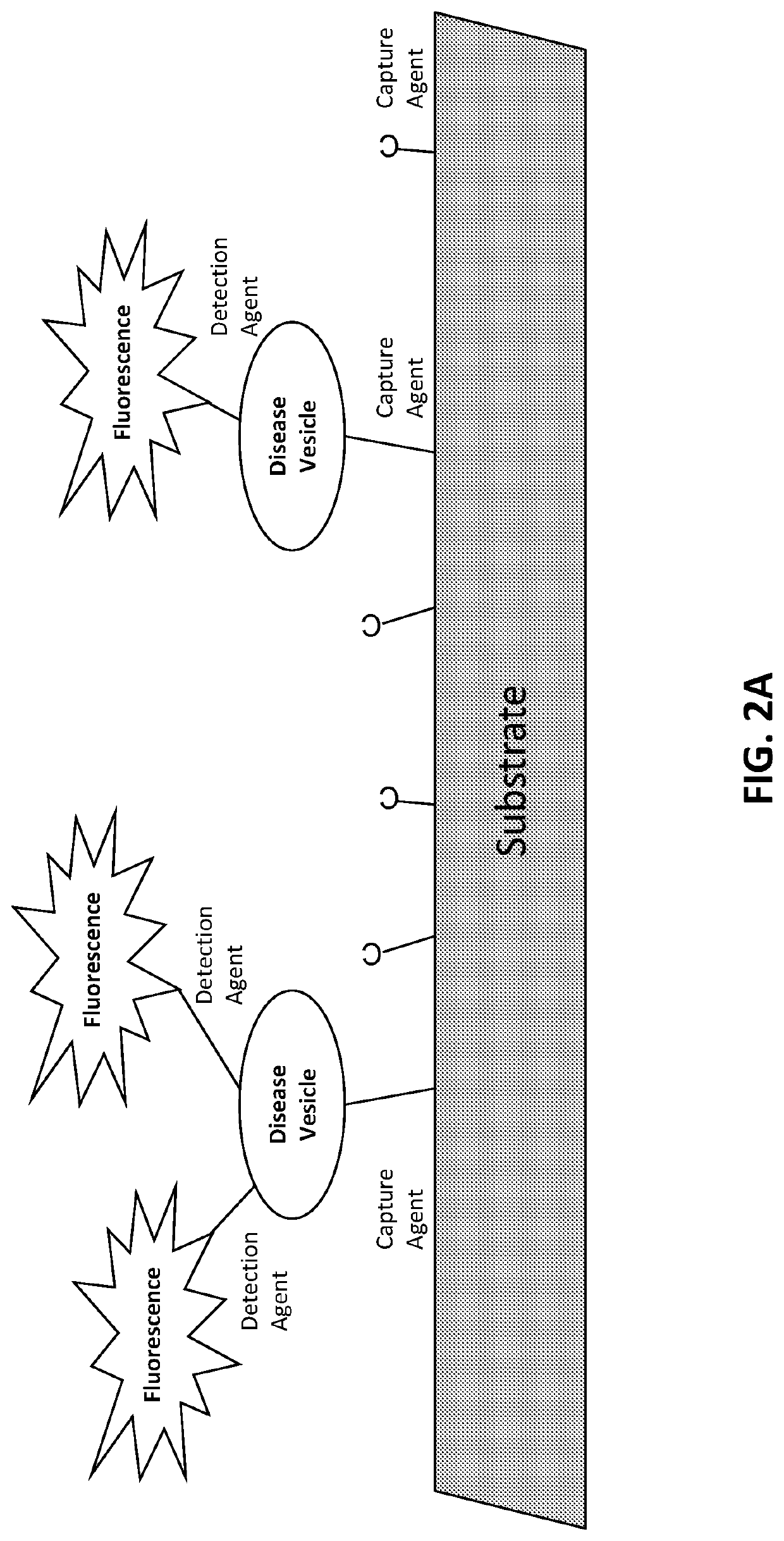
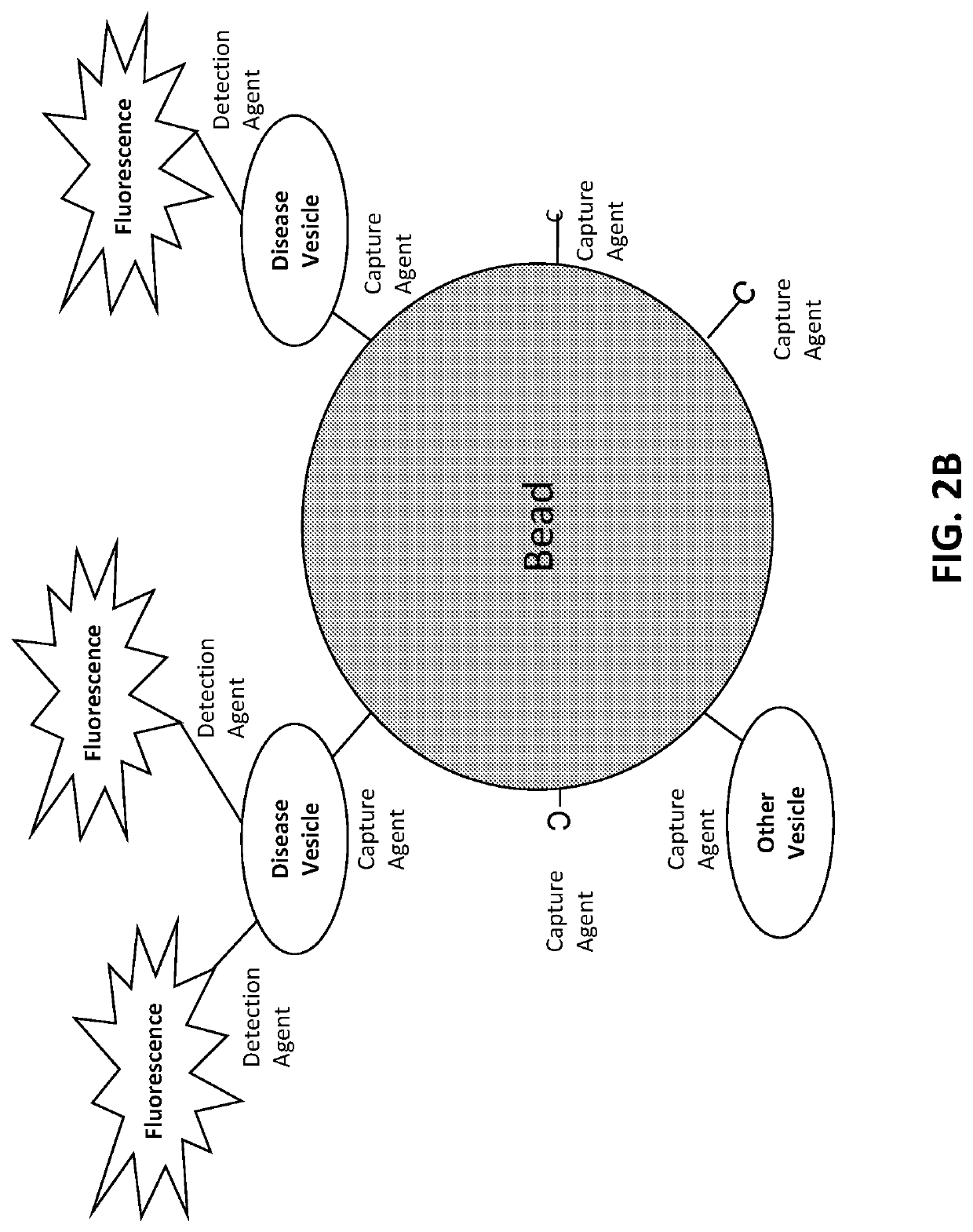
[0646]The target is affixed to a solid substrate, such as a glass slide or a magnetic bead. For a magnetic bead preparation, beads are incubated with a concentration of target protein ranging from 0.1 to 1 mg / ml. The target protein is conjugated to the beads according to a chemistry provided by the particular bead manufacturer. Typically, this involves coupling via an N-hydroxysuccinimide (NHS) functional group process. Unoccupied NHS groups are rendered inactive following conjugation with the target.
[0647]Randomly generated oligonucleotides (oligos) of a certain length, such as 32 base pairs long, are added to a container holding the stabilized target. Each oligo contains 6 thymine nucleotides (a “thymine tail”) at either the 5 or 3 prime end, along with a single molecule of biotin conjugated to the thymine tail. Additional molecules of biotin could be added. Each oligo is also manufactured with a short stretch of nucleotides on each ...
example 2
ve Assay
[0651]The process is performed as in Example 1 above, except that a known ligand to the target, such as an antibody, is used to elute the bound oligo species (as opposed to or in addition to the chaotropic agent). In this case, anti-EpCAM antibody from Santa Cruz Biotechnology, Inc. was used to elute the aptamers from the target EpCAM.
example 3
and Affinity Analysis
[0652]All aptamers generated from the binding assays described above are sequenced using a high-throughput sequencing platform, such as the Ion Torrent from Life Technologies:
[0653]Library Preparation—
[0654]Aptamers were pooled after ligating barcodes and adapter sequences (Life Technologies) according to manufacturer protocols. In brief, equimolar pools of the aptamers were made using the following steps: Analyzed an aliquot of each library with a Bioanalyzer™ instrument and Agilent DNA 1000 Kit or Agilent High Sensitivity Kit, as appropriate for the final library concentration.
[0655]The molar concentration (nmol / L) of each amplicon library was determined using the commercially available software (Agilent).
[0656]An equimolar pool of the library was prepared at the highest possible concentration.
[0657]The combined concentration of the pooled library stock was calculated.
[0658]The template dilution factor of the library pool was determined using the following equ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| kDa heat shock | aaaaa | aaaaa |
| Elongation factor | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com