Electroless copper plating compositions and methods for electroless plating copper on substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Electroless Copper Plating Rates of an Electroless Copper Plating Baths Containing Pyridinium Compounds
[0061]Ten (10) electroless copper plating baths are prepared. All ten baths include the following components:
[0062]1. 10 g / L Copper sulfate pentahydrate
[0063]2. 40 g / L Rochelle salts
[0064]3. 8 g / L Sodium hydroxide
[0065]4. 4 g / L Formaldehyde
[0066]5. 0.5 ppm 2,2′-dithiodisuccinic acid
[0067]6. Water (balance)
The pH of each bath is 13. To nine (9) of the electroless plating compositions one of the following pyridinium compounds is added in the amount specified in Table 1. Bath 10 is a control where no pyridinium compounded is added.
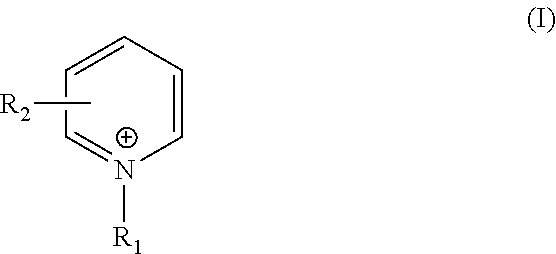
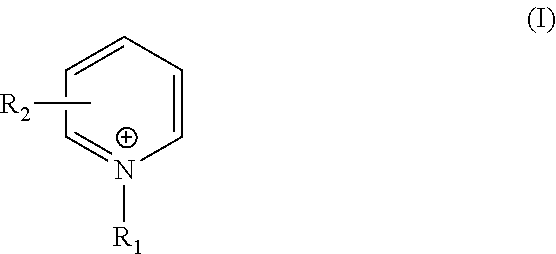
TABLE 11-(3-sulfopropyl)1-butylpyridiniumpyridinium1-(4-pyridyl)Bathchloridehydroxidepyridinium chloride12.5 ppm ——210 ppm——320 ppm——4—2.5 ppm —5—10 ppm—6—20 ppm—7——2.5 ppm 8——10 ppm9——20 ppm
Each bath is used to plate copper on bare epoxy substrates of NP140 material (Nanya, Taiwan). Each epoxy substrate is first treated according to the following process pr...
example 3
Electroless Copper Plating Rates of an Electroless Copper Plating Baths Containing Pyridinium Compounds and Guanidine Hydrochloride
[0084]Fourteen (14) electroless copper plating baths are prepared. All fourteen baths include the following components:
[0085]1. 10 g / L Copper sulfate pentahydrate
[0086]2. 40 g / L Rochelle salts
[0087]3. 8 g / L Sodium hydroxide
[0088]4. 4 g / L Formaldehyde
[0089]5. 0.5 ppm 2,2′-dithiodisuccinic acid
[0090]6. 0.36 ppm Guanidine Hydrochloride
[0091]7. Water (balance)
The pH of each bath is 13. To thirteen (13) of the electroless plating compositions one of the following pyridinium compounds is added in the amount specified in Table 4. Bath 24 is a control where no pyridinium compounded is added.
TABLE 41-(3-sulfopropyl)1-butylpyridiniumpyridinium1-(4-pyridyl)Bathchloridehydroxidepyridinium chloride112.5 ppm ——12 5 ppm——1310 ppm——1415 ppm——1520 ppm——16—2.5 ppm —17— 5 ppm—18—10 ppm—19—15 ppm—20—20 ppm—21——2.5 ppm 22—— 5 ppm23——10 ppm
Each bath is used to plate copper on...
example 5
Backlight Experiment with Aqueous Alkaline Electroless Cooper Compositions of the Present Invention Containing Pyridinium Compounds
[0101]The following aqueous alkaline electroless copper compositions of the invention are prepared having the components and amounts disclosed in Table 7 below.
TABLE 7ComponentBath 25Bath 26Copper sulfate pentahydrate10g / L10g / LRochelle salts40g / L40g / LSodium hydroxide8g / L8g / LFormaldehyde4g / L4g / L2,2′-Dithiosuccinic acid0.5ppm0.5ppmGuanidine hydrochloride0.36ppm0.36ppm1-butylpyridinium chloride10ppm—1-(3-sulfopropyl)—10ppmpyridinium hydroxideWaterTo one literTo one liter
The pH of the aqueous alkaline electroless copper compositions have a pH=13 at room temperature as measured using a conventional pH meter available from Fisher Scientific.
[0102]Six (6) different FR / 4 glass epoxy panels with a plurality of through-holes are provided: TUC-662, SY-1141, IT-180, 370HR, EM825 and NPGN. The panels are eight-layer copper-clad panels. TUC-662 is obtained from Taiwan...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com