Compositions for increasing resilience to traumatic brain injury
a technology of traumatic brain injury and composition, which is applied in the direction of drug composition, peptide/protein ingredients, nervous disorders, etc., can solve the problems of progressive neurological dysfunction, increased risk of neurodegeneration, and the estimated annual cost of tbi in the united states is $76.5 billion, so as to reduce the immunoreactivity of iba1 microglial marker, prevent neuron degeneration, and reduce the demyelination of the corpus callosum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Traumatic Brain Injury (TBI) Prevention Studies Using Immunocal®
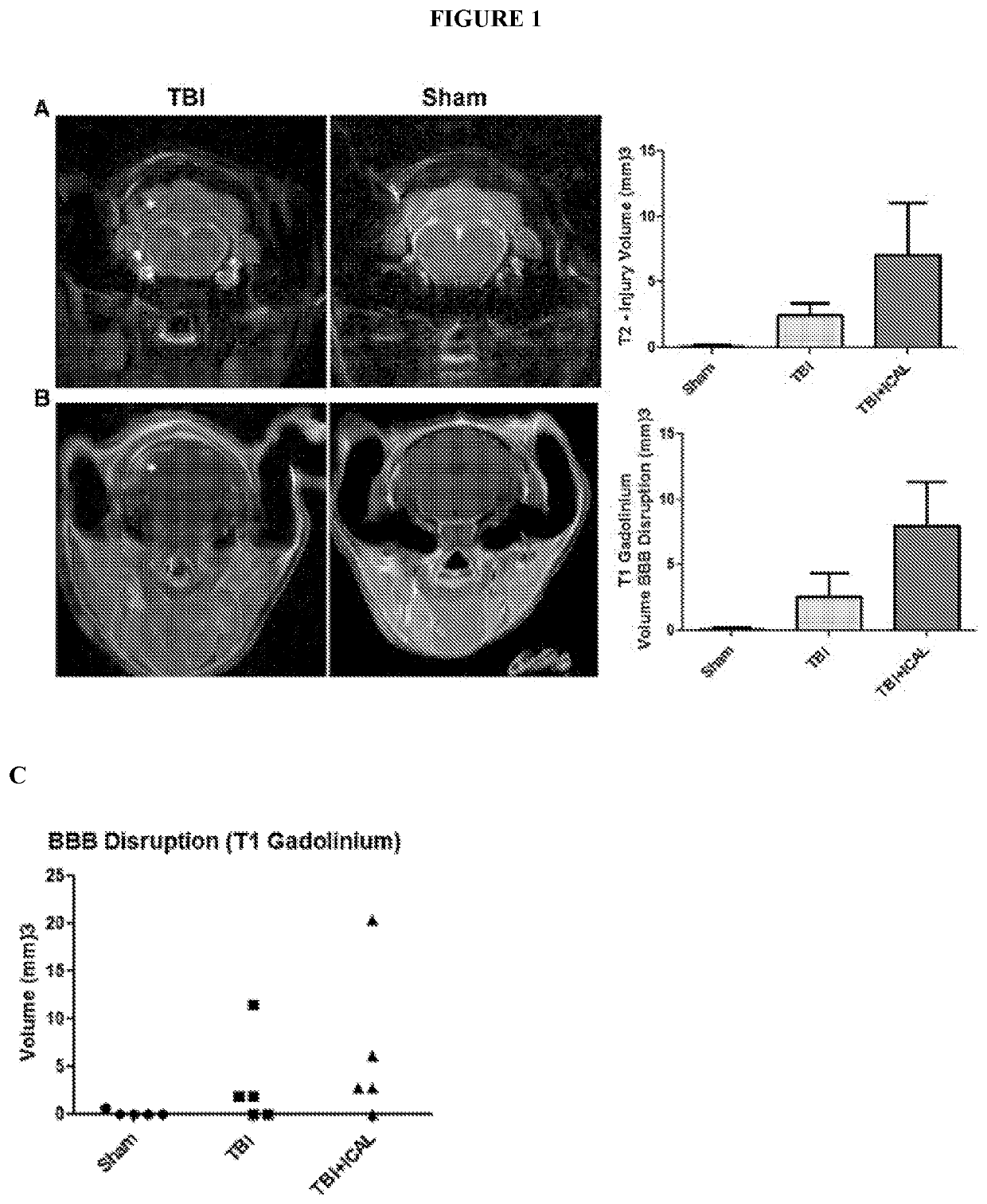
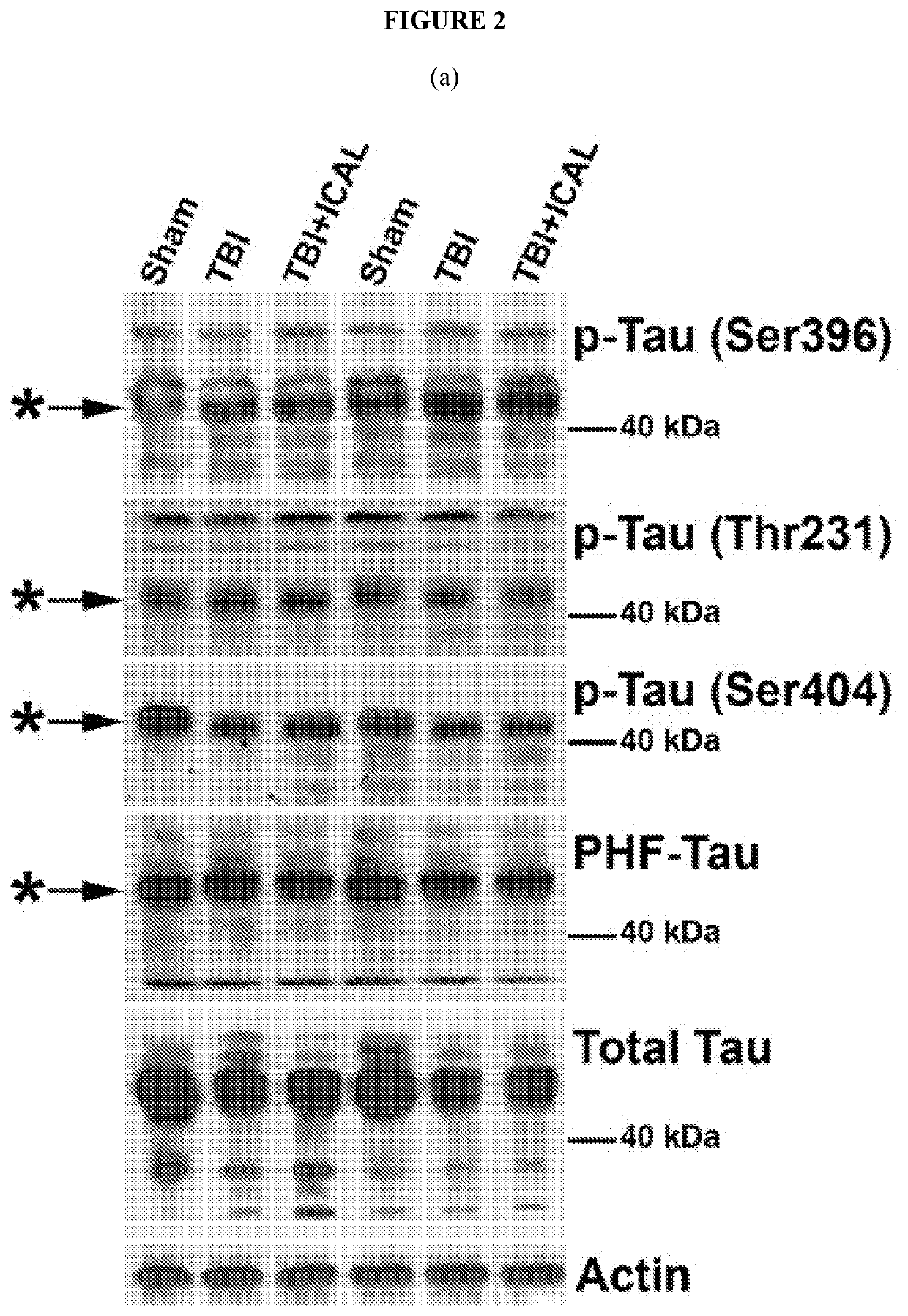
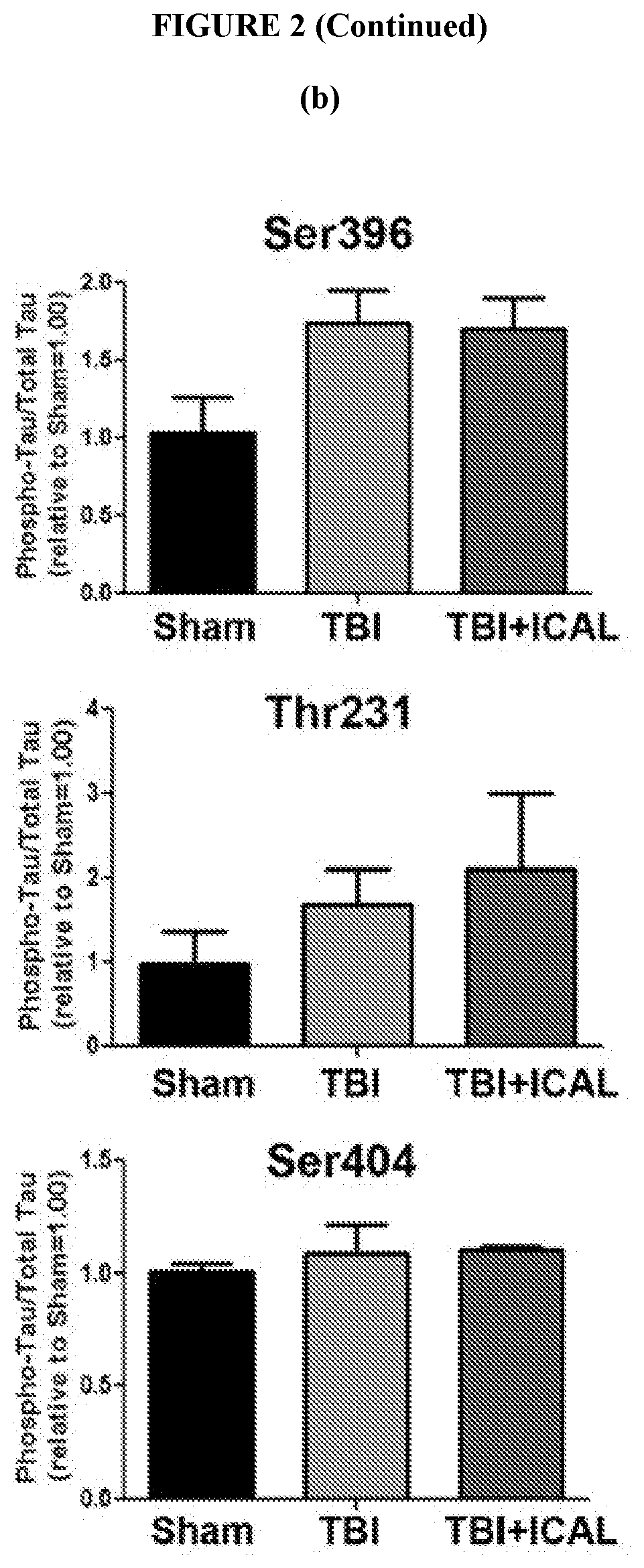
[0091]It was hypothesized that a strategy aimed at sustaining or enhancing brain GSH levels may be a viable approach to mitigate secondary injury and the subsequent long term cognitive, physical, and emotional deficiencies induced by TBI. It was further hypothesized that supplementation with whey protein isolate / concentrate such as Immunocal® prior to TBI in mice may provide enhanced resilience against oxidative damage, neuronal cell death, and / or cognitive and / or motor impairments induced by a closed head impact injury.
[0092]Accordingly, here, a whey protein supplement, Immunocal®, was tested for its potential to enhance resilience to TBI. Immunocal® is a non-denatured whey protein preparation which has been shown to act as a cysteine delivery system to increase levels of the essential antioxidant glutathione (GSH). Twice daily oral supplementation of CD1 mice with Immunocal® for 28 days prior to receiving a moderate T...
example 2
Whey Characteristics of Whey Protein Isolate Production
[0130]An example of whey protein isolate production is provided below for illustrative purposes intended for the person of skill in the art.
[0131]As will be understood, whey may be considered as a by-product of cheese or of casein manufacture. Whey typically contains soluble proteins of milk, so-called whey proteins. Cheese whey, for example, typically contains 5-8 g / l of proteins (N×6.38), among which β-lactoglobulin (β-lg) and α-lactalbumin (α-la) are the most abundant (accounting for 50-55% and 15-20% of total whey proteins, respectively) and bovine serum albumin (BSA), lactoferrin (LF) and immunoglobulins (IgG) are considered as minor whey proteins (accounting each for 3-5%). Whey may also comprise protein fragments or polypeptides such as so-called proteose-peptones (PP-4, PP-5, PP-8f) resulting from proteolysis of milk proteins by lactic starters in cheesemaking or by psychrotrophic bacteria during cold storage of raw milk...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| wt % | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com