Balloon assisted endoluminal prosthesis deployment
a technology of endoluminal prosthesis and balloon, which is applied in the field of balloon assisted endoluminal prosthesis deployment, can solve the problems of large lateral stiffness, high morbidity and mortality rate of procedures to treat aortic aneurysms, and generally regarded as involving higher risk and more difficulty, and achieves sufficient column strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 10
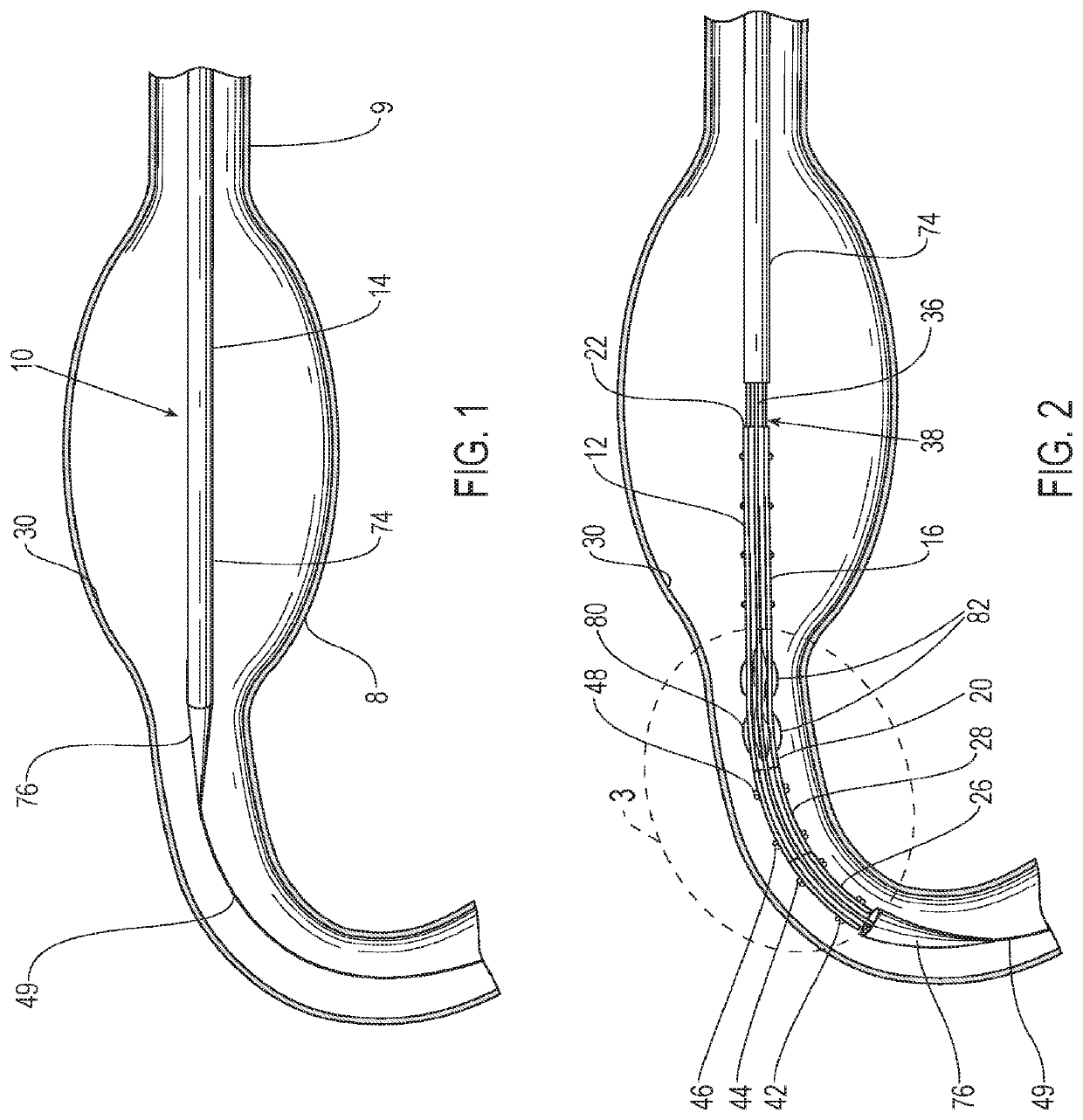
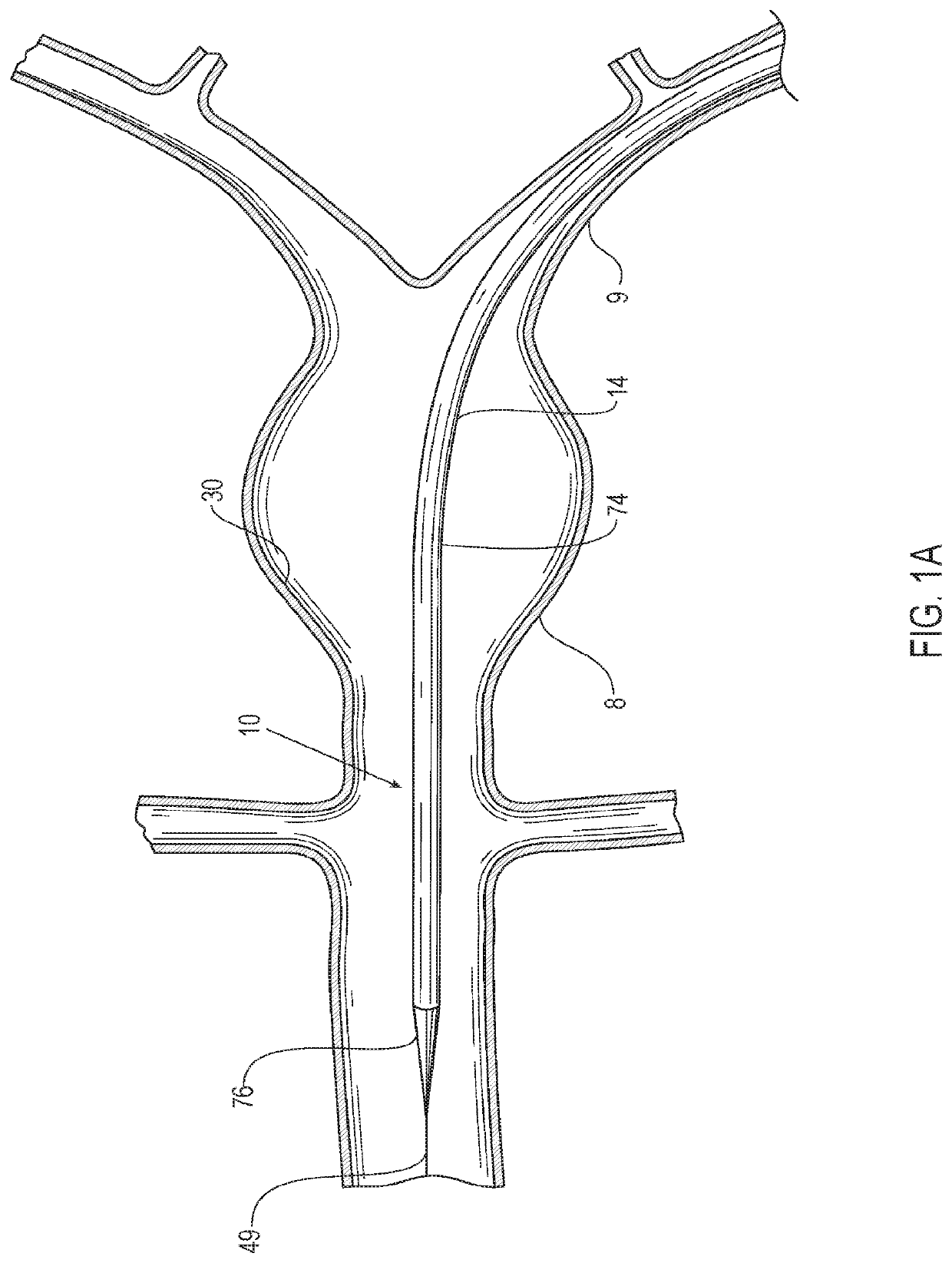
[0049]Referring to the figures, an embodiment of a delivery system 10 for treatment of a vascular defect such as aneurysm 8 is shown in FIGS. 1-12. Although the exemplary aneurysm 8 shown in the patient's vasculature 9 is a thoracic type aortic aneurysm, devices and methods as discussed herein and illustrated in the corresponding figures may also be used for any other suitable type of vascular defect such as abdominal aortic aneurysms (shown in FIG. 1A) and the like. The delivery system embodiment 10 may include an endoluminal prosthesis 12 for treatment of the vascular defect 8 and a delivery catheter 14. The endoluminal prosthesis may include a tubular main graft portion 16 made from a thin flexible material, and including a main inner lumen 18, a proximal end 20 and a distal end 22. The graft body may be formed from a flexible and supple graft material, such as PTFE, and have a main fluid flow lumen disposed in a main graft portion therein. For some embodiments, flexible graft ma...
embodiment 24
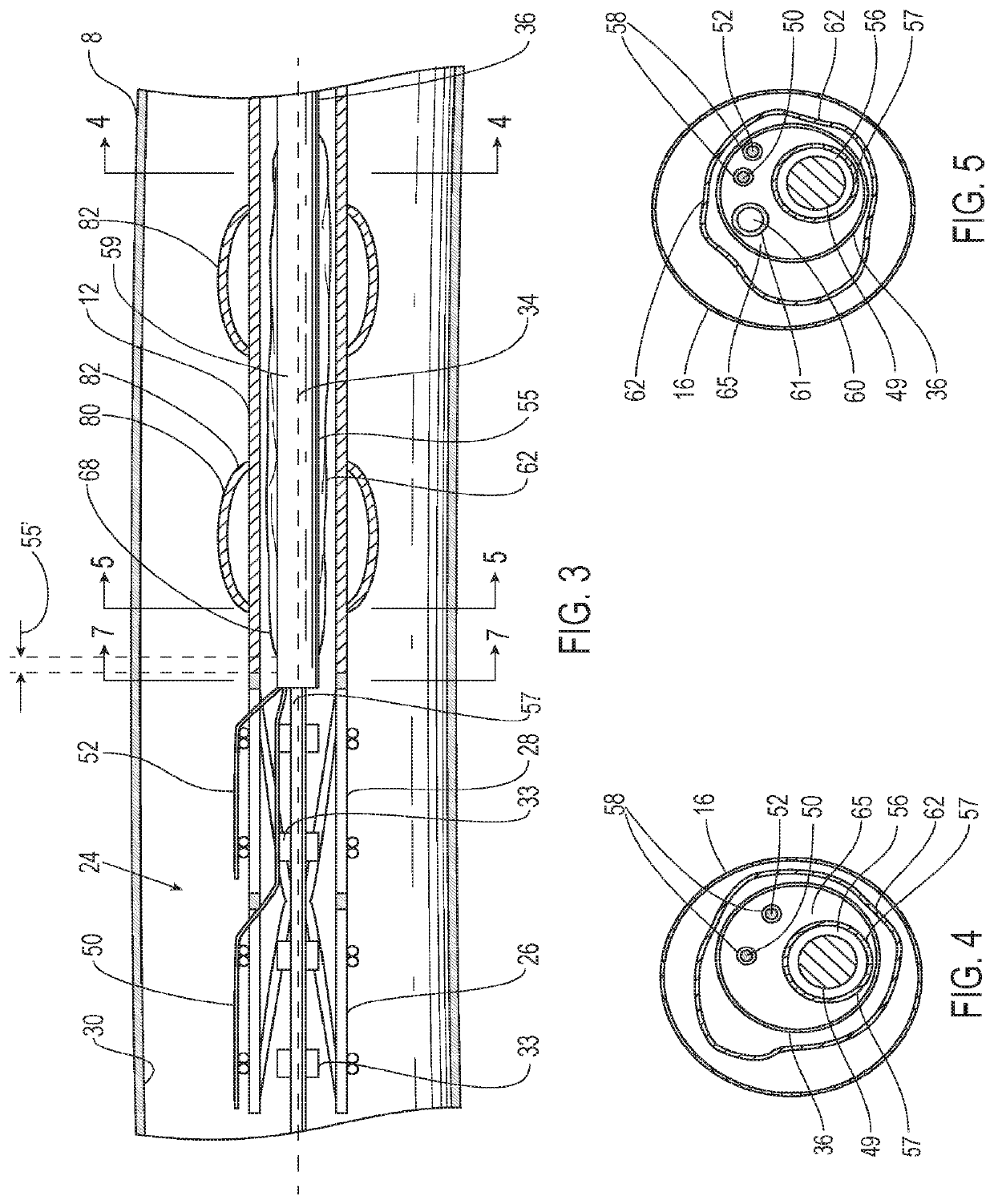
[0050]Referring to FIG. 3, the endoluminal prosthesis 12 may also have a self-expanding anchor member 24 that has a proximal portion 26 and a distal portion 28 with a distal end of the distal portion being secured to a proximal end 20 of the tubular main graft portion and a distal end of the proximal portion being secured to a proximal end of the distal portion. The self-expanding anchor member embodiment 24 shown in FIG. 2 has a generally cylindrical configuration with a free unsecured end at a proximal end thereof and a distal end that is secured to a proximal end of the main graft body. The proximal portion 26 of the self-expanding anchor member 24 may include a cylindrical stent including an elongate superelastic element disposed in a zig-zag configuration and the distal portion of the self-expanding anchor member comprises a cylindrical stent including an elongate superelastic element disposed in a zig-zag configuration. An optional connector ring (not shown) may be embedded in...
embodiment 14
[0059]In some instances, the friction generated by axial movement of the first and second release members 50, 52 may be minimized by using a multi-lumen configuration in the elongate shaft 36 of the delivery catheter 14. Such a delivery catheter embodiment 14 may include an elongate shaft 36 with one or more release member lumens 58 extending within a release member sleeve 67, a guidewire lumen 56 extending within a guidewire tube 57 for passage of a guidewire 49, an inflation lumen 60 for inflation of an inflatable balloon 62 extending within an inflation tube 61 and an optional fill lumen 64 for filling an optional inflatable portion of the endoluminal prosthesis 12 extending within a fill tube 69. The section of the elongate shaft 36 of the delivery catheter embodiment 14 shown in FIG. 5 illustrates the release member lumens 58 and guidewire lumen 56, surrounded by guidewire tube 57. The section in FIG. 4 also includes the inflation lumen 60 surrounded by the inflation tube 61.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com