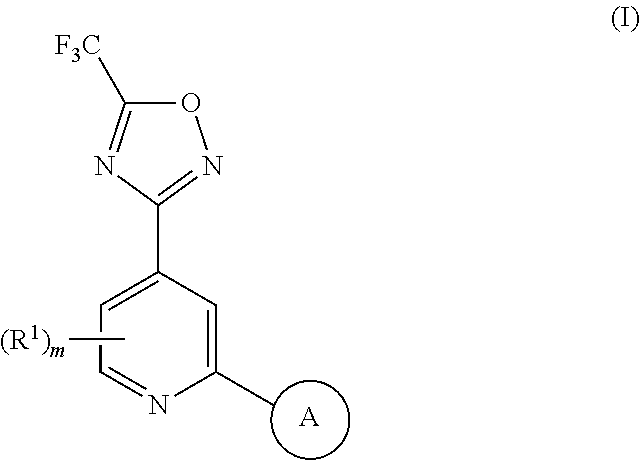
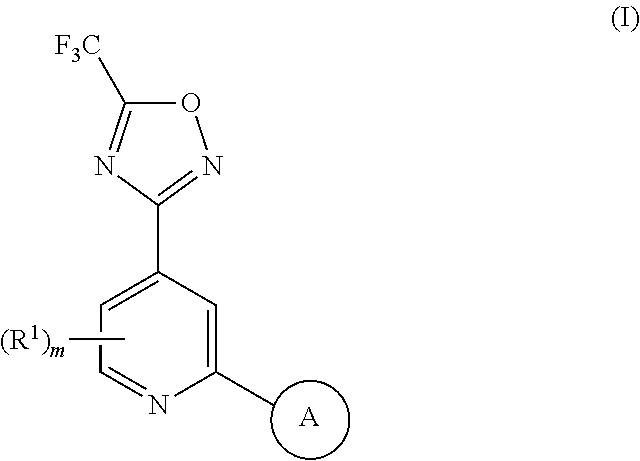
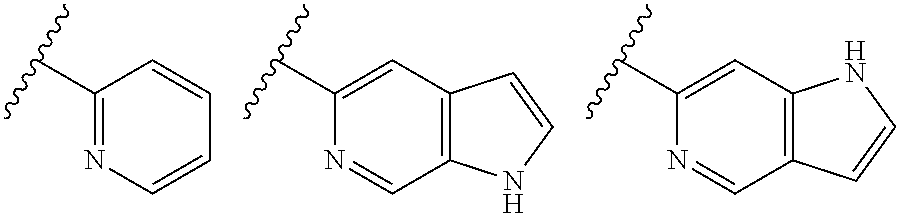
1,2,4-oxadiazole derivatives as histone deacetylase 6 inhibitors
a technology of histone deacetylase and derivatives, which is applied in the field of 1, 2, 4oxadiazole derivatives, can solve the problems of toxic side effects and significant side effects of pan-hdac inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
reference example 1
2-(Trimethylstannyl)isonicotinonitrile
[0321]To a stirred solution of 2-bromoisoniconitrile (2 g, 10.92 mmol) in toluene (20 mL), hexamethylditin (4.6 g, 14.20 mmol), and Pd(PPh3)4 (1.2 g, 1.09 mmol) were added at rt. The resulting solution was degassed with nitrogen for 10 min and heated to 110° C. for 16 h. The reaction mixture was evaporated under reduced pressure and the crude compound was purified by flash column chromatography on neutral alumina using 50% EtOAc in petroleum ether to afford the title compound (1.5 g, 51.7%).
[0322]LC-MS (method 1): Rt=1.93 min; m / z=269.08 (M+H+)
reference example 2
5-Bromo-1-butyl-1H-pyrrolo[2,3-c]pyridine
[0323]To a stirred suspension of 60% NaH (0.146 g, 6.091 mmol) in DMF (20 mL), 5-bromo-1H-pyrrolo[2,3-c]pyridine (0.8 g, 4.06 mmol) was added at 0° C. and stirred for 15 min. Then, 1-bromo butane (0.66 g, 4.873 mmol) was added to the reaction mixture at 0° C. The resulting mixture was allowed to warm to rt and stirred for 16 h. The reaction mixture was quenched with water and extracted EtOAc and the organic layer was dried over anhydrous Na2SO4, and it was concentrated under reduced pressure. The crude compound was purified by flash column chromatography using 10% EtOAc in pet ether as an eluent to afford the title compound (0.78 g, 67%).
[0324]LC-MS (method 2): Rt=2.27 min; m / z=253.17 (M+H+).
[0325]Following a similar procedure to that described in reference example 2, but using in each case the corresponding starting materials, the following compounds were obtained:
ReferenceStartingHPLCRt exampleCompound namematerialmethod(min)m / z2a5-Bromo-1-...
reference example 3
5-Bromo-1-butyl-N,N-dimethyl-1H-pyrrolo[2,3-c]pyridine-2-carboxamide
Step a. 5-Bromo-N,N-dimethyl-1H-pyrrolo[2,3-c]pyridine-2-carboxamide
[0326]To a stirred solution of 5-bromo-1H-pyrrolo[2,3-c]pyridine-2-carboxylic acid (500 mg, 2.07 mmol) in DMF, dimethyl amine hydrochloride (168 mg, 2.07 mmol), TEA (1.49 g, 10.37 mmol) and T3P (1.97 g, 6.21 mmol) were added at 0° C. The resulting mixture was allowed to stir at rt for 16 h. The reaction mixture was diluted with water and extracted with EtOAc. The combined organic layers were dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The crude compound was purified by silica gel column chromatography and eluted at 5% MeOH in DCM to afford the title compound (450 mg, 78%).
[0327]LC-MS (method 4): Rt=1.75 min; m / z=270.15 (M+H++2).
Step b. 5-Bromo-1-butyl-N,N-dimethyl-1H-pyrrolo[2,3-c]pyridine-2-carboxamide
[0328]To a stirred solution of the compound obtained in the previous section, step a (1.4 g, 5.24 mmol) in DMF, 60...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com