Compositions and Methods for Treating Pain with Wogonin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
reatment of a Mouse Model of Osteoarthritis Formulated as a Cream
[0083]The use of wogonin for the treatment of osteoarthritic (OA) pain was investigated. Specifically, the benefit of using small doses of wogonin applied topically in a cream with penetration enhancers was investigated. The cream formulation shown in Table 2 was used in this study. An additional exemplary cream formulation for administering wogonin is provided in Table 3.
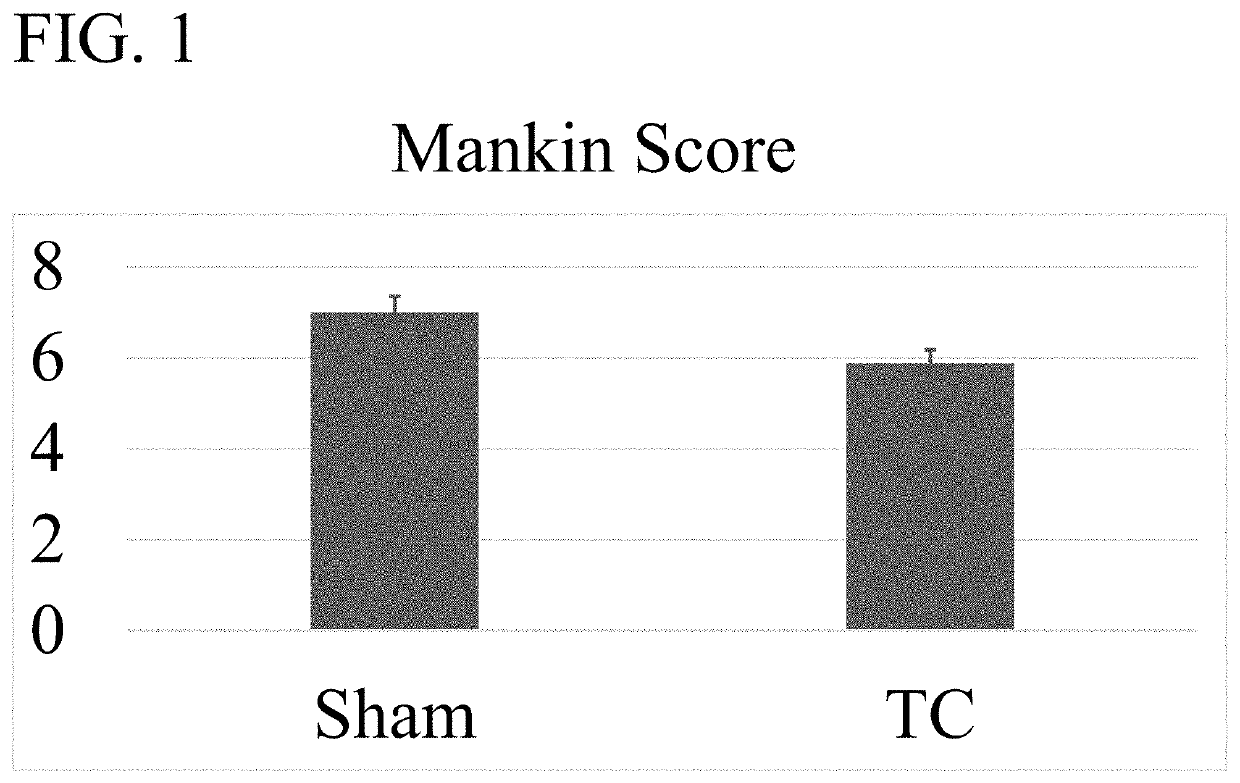
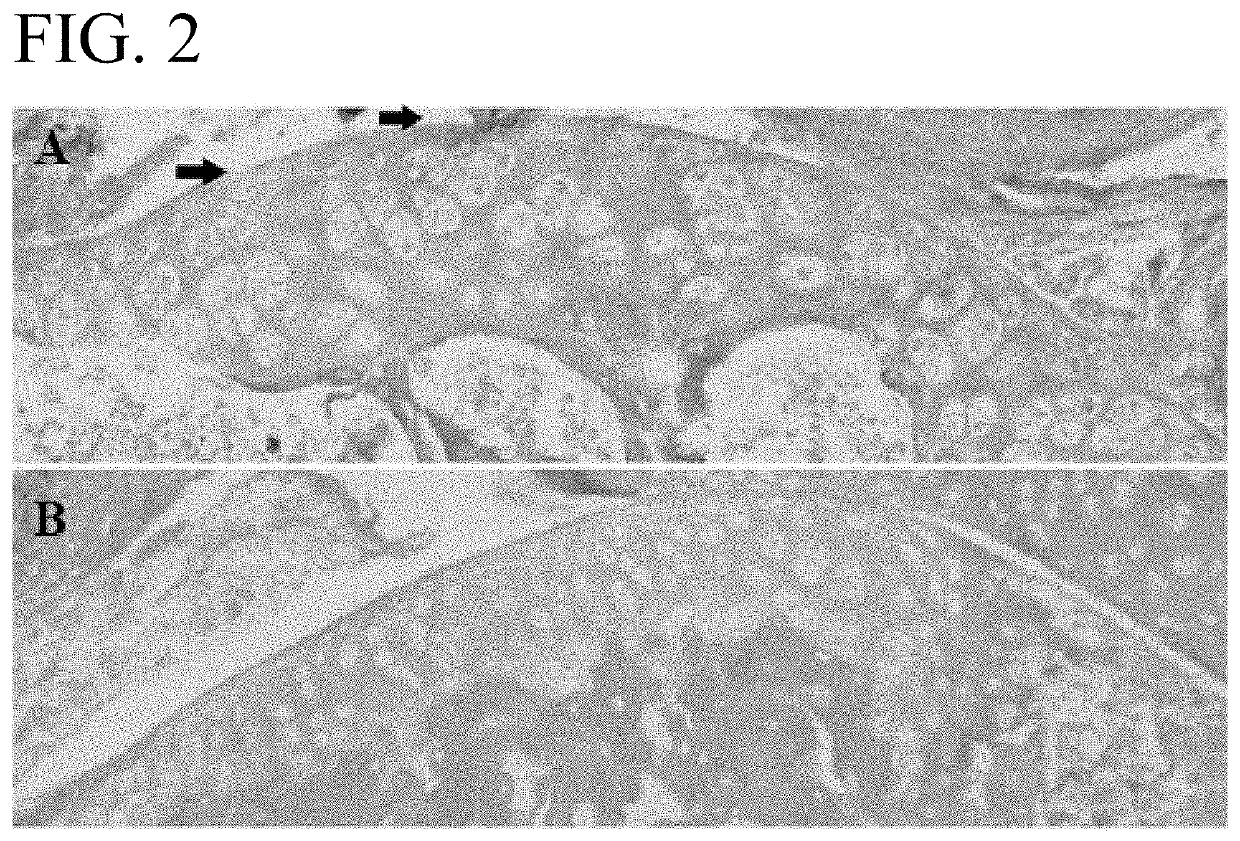
[0084]An OA mouse model was used to determine the efficacy of wogonin treatment. OA-like disease was induced by surgical destabilization of the medial meniscal ligament (DMM) of laboratory mice. Repair of the knee was assessed using the Modified Mankin Score which is widely accepted as the way to objectively compare OA progression by histological evaluation of a knee joint (See Larkin D J et al., Frontiers in physiology. 2013; 4:121 and Mankin H J. The New England journal of medicine. 1974; 291(24):1285-92). The mice were housed in activity wheel cage...
example 2
reatment of a Mouse Model of Osteoarthritis Formulated in a Cream or in a Liquid
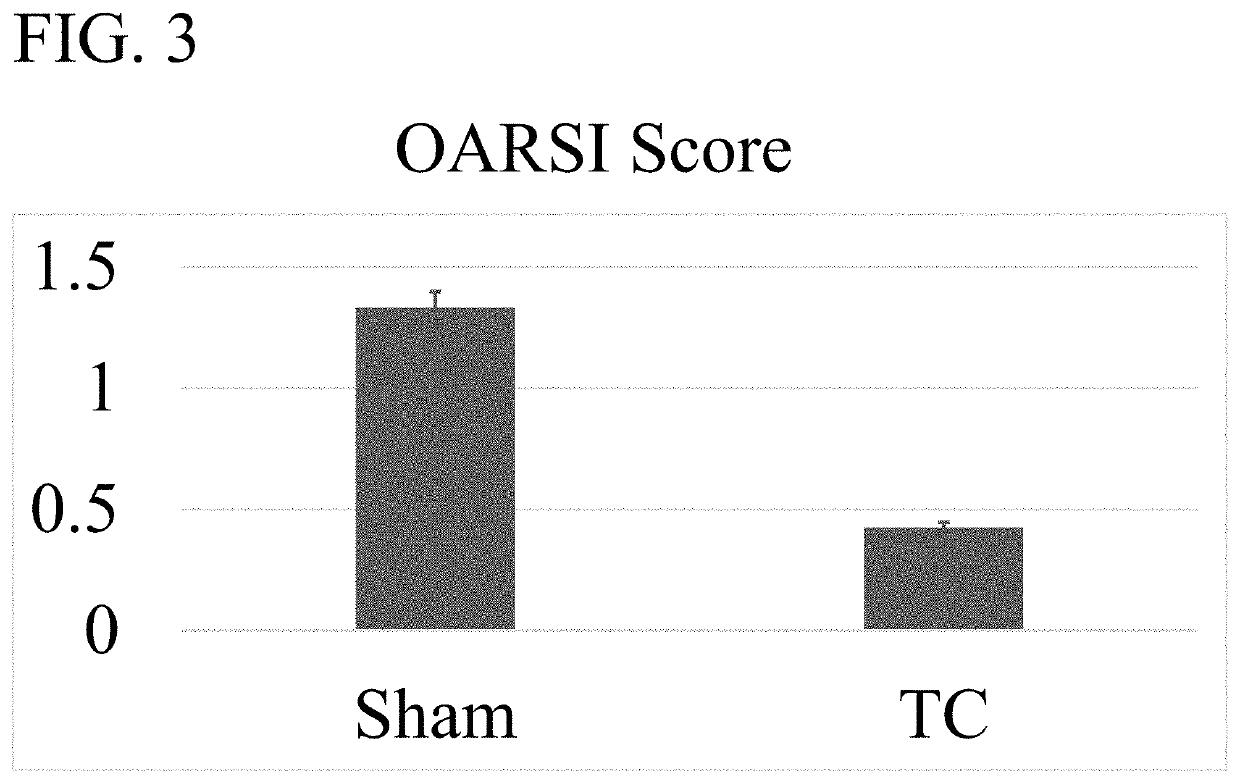
[0089]A second experiment using small doses of wogonin applied topically either in DMSO or in a cream with penetration enhancers was conducted. The cream formulation provided in Table 1 was used. In this study, OA was induced by DMM surgery. Twenty four hours after surgery treatment was applied to the affected knee. The treatments included 0.00000142 g wogonin in 10 μl DMSO (500 μM wogonin) applied to the skin at the location of patellar tendon (n=5); 10 μl DMSO applied to the skin at the location of patellar tendon (n=5); 0.0000000028 g wogonin (10 μl G2 Cream E / 10 μM wogonin) to the skin at the location of the patellar tendon (n=5). Treatment was applied every third day for 28 days. Immediately after surgery, mice were placed in an activity wheel cage in which wheel use was recorded on a computer. Seven days after starting the experiment, the mice treated receiving either wogonin in DMSO or in a cream ...
example 3
ormulation for the Administration of Wogonin
[0091]An exemplary roll-on formulation for the administration of wogonin is provided in Table 6.
TABLE 6Exemplary Roll-on CompositionIngredientWaterAlcoholCaprylic / Capric TriglyceridePropanediolEthoxydiglycolMentha Piperita (Peppermint) OilDimethiconeCetearyl AlcoholGlyceryl StearatePotassium Olivoyl Hydrolized Oat ProteinCetyl AlcoholXanthan GumPhenoxyethanolCaprylyl GlycolGlyceryl OleateSclerotium GumLecithinEthylhexylglycerinHexylene GlycolPullulanMentholSodium PhytateWogonin
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com