Catalyst compositions for hydroformylation and methods of use thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
droformylation of 1-Alkenes
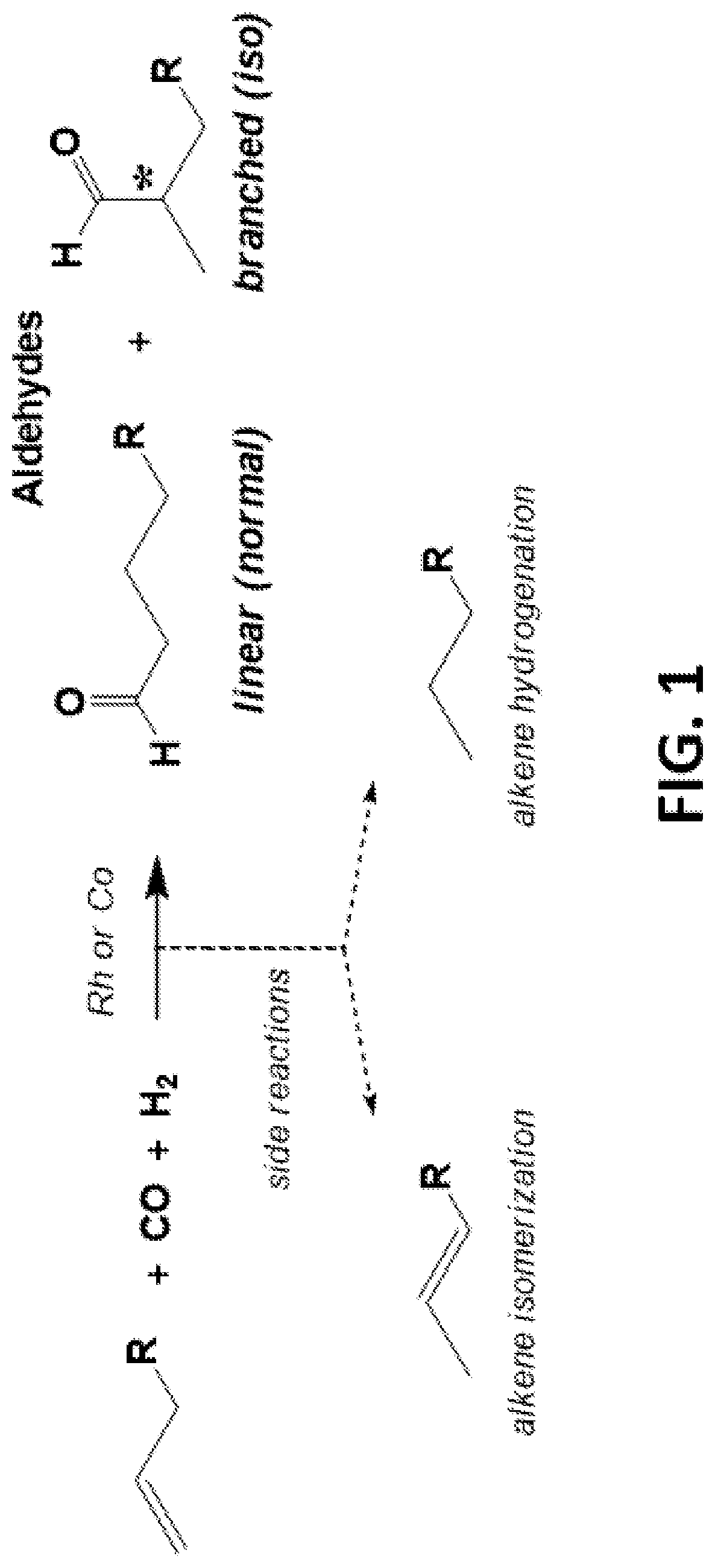
[0131]Cationic monometallic and bimetallic bis(phosphine)-chelated cobalt catalysts that perform hydroformylation under far lower temperatures and pressures than known systems using neutral cobalt catalysts, and with far higher activities, have been identified and are described herein. An exemplary hydroformylation reaction is shown in FIG. 1. The catalysts of the present disclosure are also active in alkene isomerization with little alkene hydrogenation observed, which is desirable for current processes. The catalysts partially convert aldehydes generated from hydroformylation into desired alcohol products in the presence of hydrogen. Though linear to branched (L:B) regioselectivities of 0.8:1 to 1.4:1 were observed in the aldehyde products derived from 1-alkenes, the chelating bisphosphine ligand may be modified to yield higher aldehyde L:B ratios with 1-alkenes.
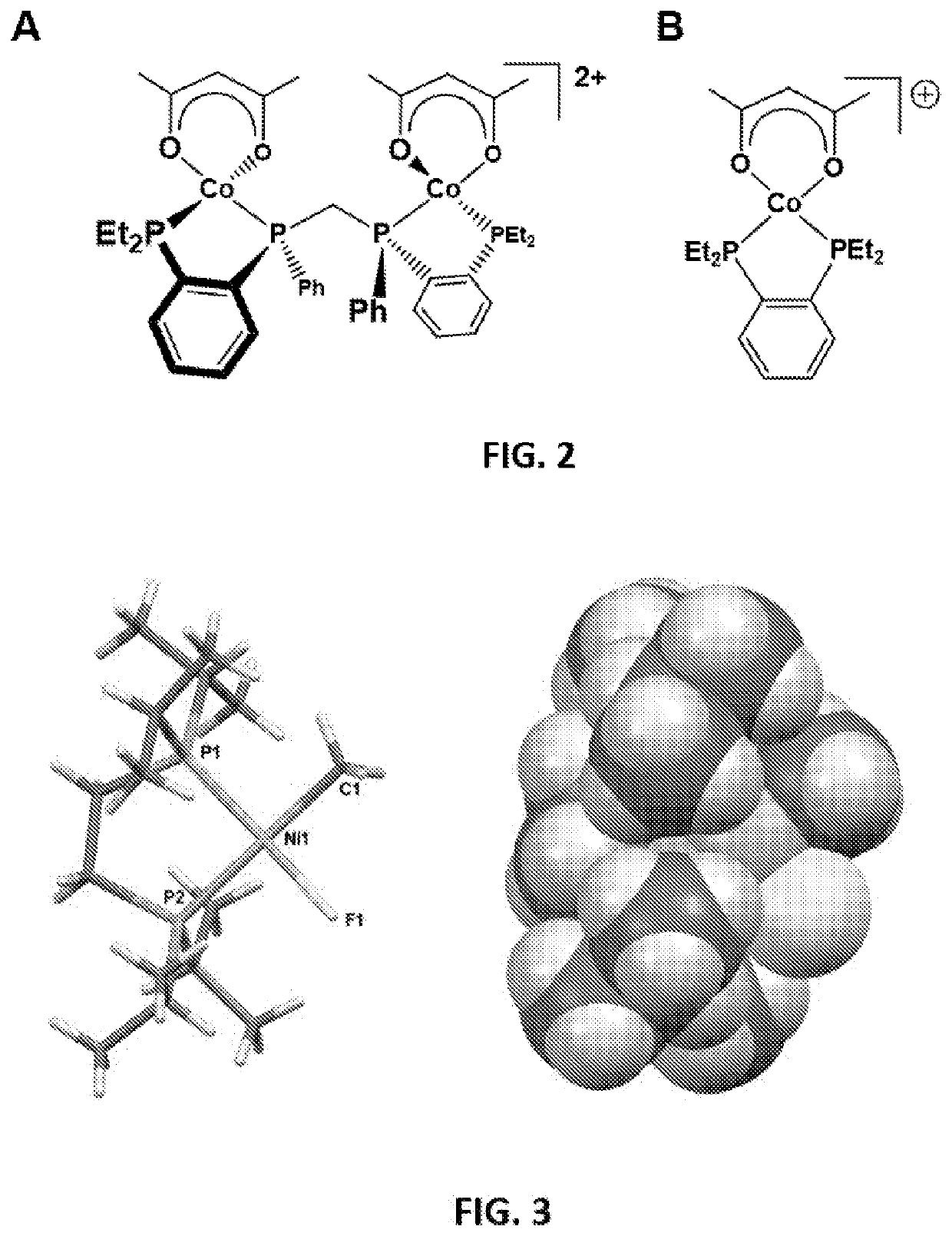
[0132]The development of dicationic dicobalt catalysts are based on the stronger coordinating...
example 2
droformylation of Internal Branched Alkenes
[0135]The cationic cobalt catalysts described herein have excellent activity and much higher L:B selectivity for difficult to hydroformylate internal branched alkenes like 2-methyl-2-butene. Hydroformylation runs with 2-methyl-2-butene were done with [Co(acac)(dppe)](BF4), dppe=Et2PCH2CH2PEt2, and Rh(acac)(CO)2+PPh3. The following reaction conditions were utilized: (a) HRh(CO)(PPh3)2: 1 mM Rh(acac)(CO)2, 0.4 M PPh3, 400:1 PPh3:Rh, 1 M 2-methyl-2-butene, 100° C., 7.9 bar, 1:1 H2 / CO in toluene; and (b) [HCo(CO)(dppe)](BF4): 1 mM [Co(acac)(dppe)](BF4), 1 M 2-methyl-2-butene, 140° C., 34.5 bar, 1:1 H2 / CO in t-glyme. Using the foregoing, it was observed that there was no hydroformylation activity by the industrial Rh / PPh3 catalyst, no observed alkene isomerization and no alkene hydrogenation. The cationic cobalt-dppe catalyst did 286 turnovers after 3 hours (28.6% conversion) with 11:1 L:B (based on NMR and GC / MS). Less than 1% alkene hydrogenat...
example 3
ative Active Catalysts
[0136]A variety of chelating bisphosphine ligands were examined for their effect on the hydroformylation activity and selectivity of the monometallic cationic Co(II) catalyst system (BF4− counter-anion, other non-coordination anions should work well). The initial hypothesis was that the extremely strong chelate effect of 1,2-phenylene-linked bisphosphines was critically important in stabilizing the low-spin, cationic Co(II) oxidation state; however, further studies demonstrated that other chelating bisphosphine ligands work well. The strength of the bisphosphine chelate, is clearly important for the overall catalyst stability, but the effect of the phosphine R-groups is even more dramatic.
[0137]The following phosphines have been tested using the cationic cobalt(II) acac catalyst precursor motif. The most successful ligands, which have a bridging 1,2-phenylene or saturated alkyl group, generate active cationic Co(II) hydroformylation catalysts. All have similar ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com