Method of producing pharmaceutical compositions comprising immunogenic chikungunya virus chikv-delta5nsp3
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
rials for CHIKV-Δ5nsP3 Drug Substance (DS) Production
[0056]Assembly of Synthesized CHIKV-Δ5nsP3 Genome
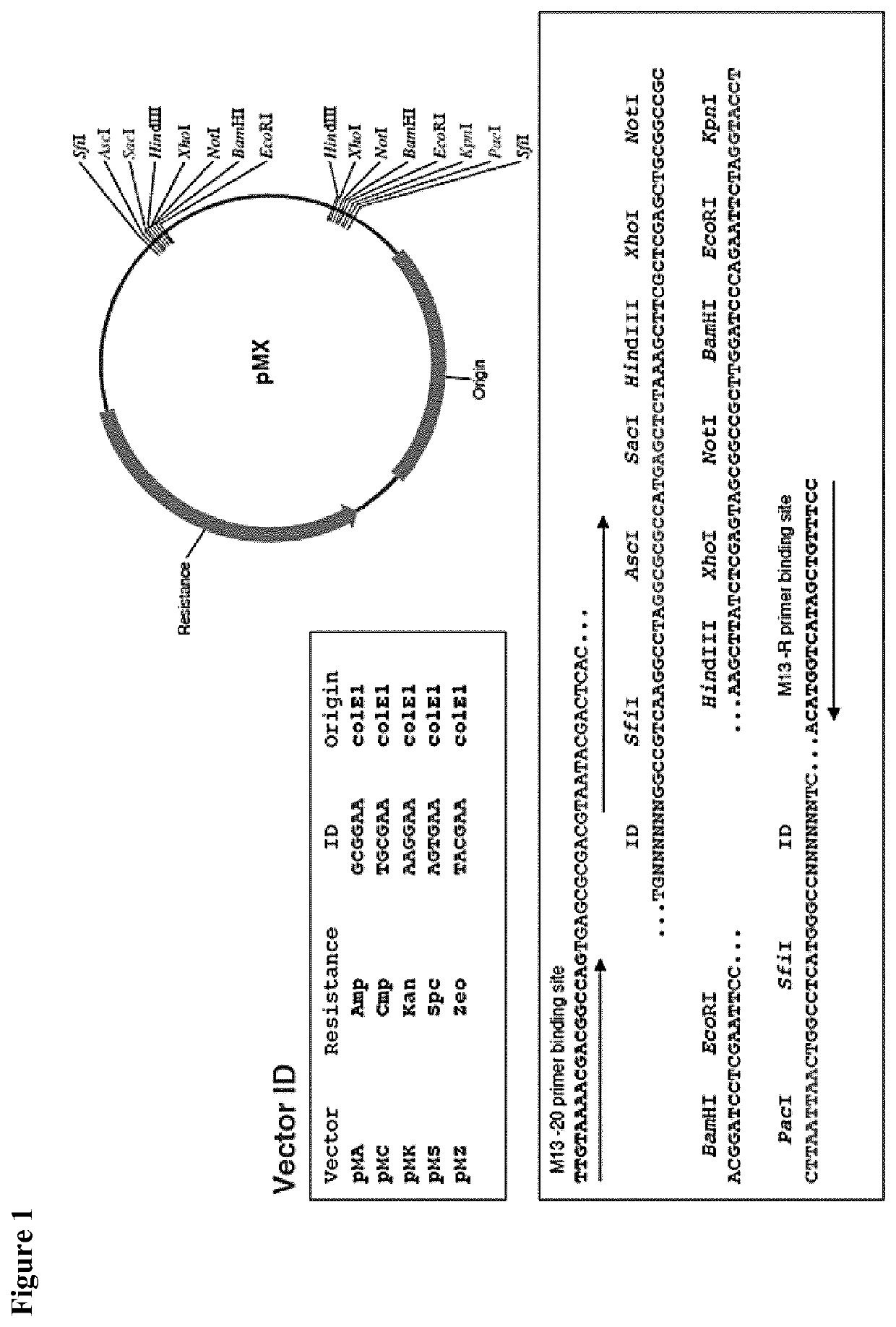
[0057]The CHIKV-Δ5nsP3 virus genome was synthesized in five fragments at MWG Eurofins (Germany) and was fully assembled in the pMA plasmid (pMX vector with ampicillin resistance), a standard cloning vector. The pMX vector backbone is shown in FIG. 1. All cloning and plasmid preparation procedures were carried out under TSE-free conditions using electro-competent NEB1013 E. coli cells. The cloning strategy used for the full assembly of the CHIKV-Δ5nsP3 genome in pMA is outlined in FIG. 3B. Briefly, the pMA plasmid containing fragment 1, which covers nsP1 and part of nsP2 (as shown in FIG. 3A), was linearized via EcoRI / PacI restriction digestion and fragment 2 covering parts of nsP2 and nsP3 was fused to fragment 1. In parallel, fragment 3 (covering nsP4 and C) was fused to fragment 4 (covering C and E2) via ClaI / PacI cloning. In a third cloning step, fragments 3 and 4 were cloned via...
example 2
Sequence Heterogeneities of CHIKV-Δ5nsP3 which Affect Immunogenicity
[0069]Due to the observed reduction / loss of immunogenicity (neutralizing antibody titer) and decreased plaque size at higher CHIKV-Δ5nsP3 passages, it was of interest to analyze possible sequence heterogeneities within the viral populations at different passage numbers. In addition, it was of interest to analyze the sequence of individual plaques of the viral population. Unpassaged CHIKV-Δ5nsP3 (P0) did not show sequence heterogeneities based on Sanger sequencing. In general, with increased passage numbers an increase in sequence heterogeneities for all 3 replicates was observed (Replicates A, B and C; Table 3). In the case of passaging replicate C at passage 8 (P8C), the virus population was still heterogeneous (sequence heterogeneities shown in Table 3), whereas the P15C passage showed a more homogenous virus population with defined point mutations (indicated by *). The immunogenicity data shown in FIG. 4C (Exampl...
example 3
Sequence Heterogeneities and Immunogenicity of CHIKV-Δ5nsP3 at Passage P3
[0080]The occurrence at later passages of sequence heterogeneities with adverse effects on the immunogenicity of CHIKV-Δ5nsP3 as measured by neutralizing antibody titers warranted finding the optimal passage which was characterized by both high immunogenicity as well as a viral titer sufficient for production of an effective vaccine.
[0081]To determine genetic stability of the CHIKV-Δ5nsP3 during MVSB (P1), WVSB (P2) and CHIKV-Δ5nsP3 drug substance (“VLA1553”) (P3) production, independently generated passages 1, 2 and 3 were sequenced. As determined by Sanger sequencing, P0 (virus rescue), P1 (MVSB) and P2 (WVSB) did not show any obvious sequence heterogeneities. The next step was to demonstrate reproducibility of genetic stability of P3 derived purified drug substance (DS) using P2 (WVSB) for infection. In total, four independent P3 harvests, consisting of combined day 1 and day 2 harvests, were produced in two...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com