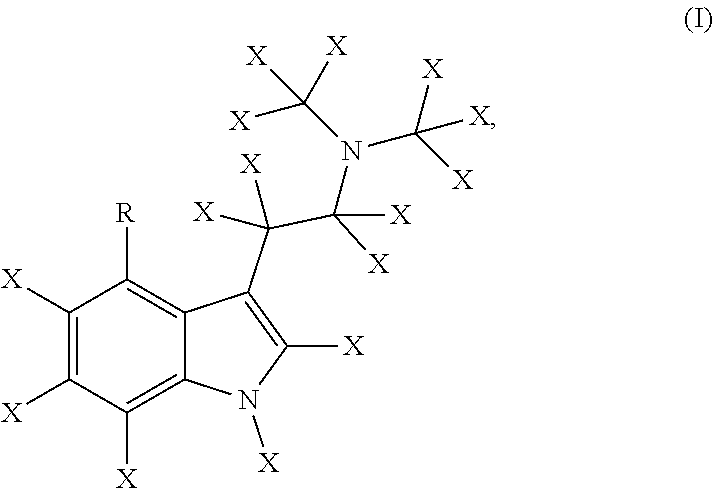
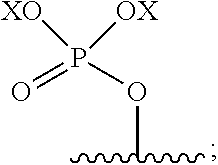
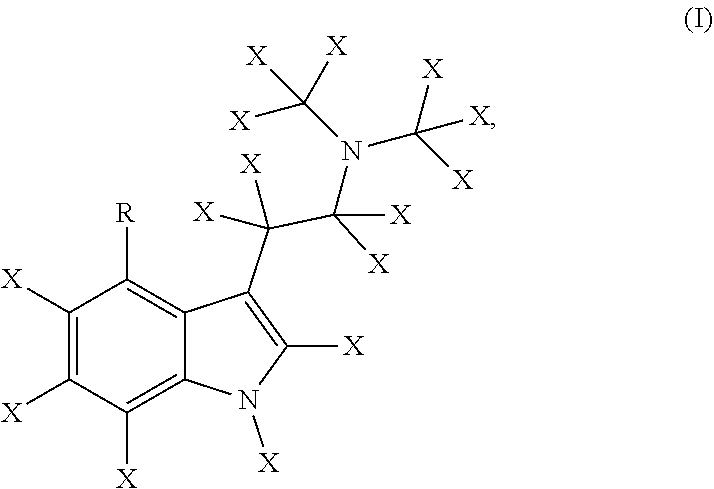
Deuterated derivatives of psilocybin and uses thereof
a technology of psilocybin and deuterated derivatives, which is applied in the field of deuterated derivatives of psilocybin and its use, and achieves the effects of increasing the duration of action, increasing blood pressure, and increasing the heart ra
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0134]A study was conducted to assess the metabolic stability and metabolite formulation of psilocin and d10-psilocin (the compound of Formula (IB-1)) in human hepatocytes under the conditions set forth in Table 1.
TABLE 1Study speciesHuman (mixed gender, lot HQE, viability85%)Incubation volume300 μl, pH 7.4 Celsis IVT In Vitro GROKHB mediumSampling volume40 μL Cell viability1.0 million viable cells / mlDMSO content0.5%Incubation time0, 10, 20, 40 and 60 min @ 37° C.Replicates2 Termination of incubations2 - fold volume of cold 75% acetonitrileStorage of the samplesImmediate analysisTest compound concentration1 μMControlVerapamil
[0135]The results of the study are as follows. The half-lives of psilocin and d10-psilocin were calculated to be 178 minutes vs. 298 minutes, respectively. For psilocin, approximately 78% of parent remained after the 60-minute incubation, whereas for d10-psilocin approximately 84% of parent remained after the 60-minute incubation. The major metabolite for both...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| psychiatric disorder | aaaaa | aaaaa |
| plasma half-life | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com