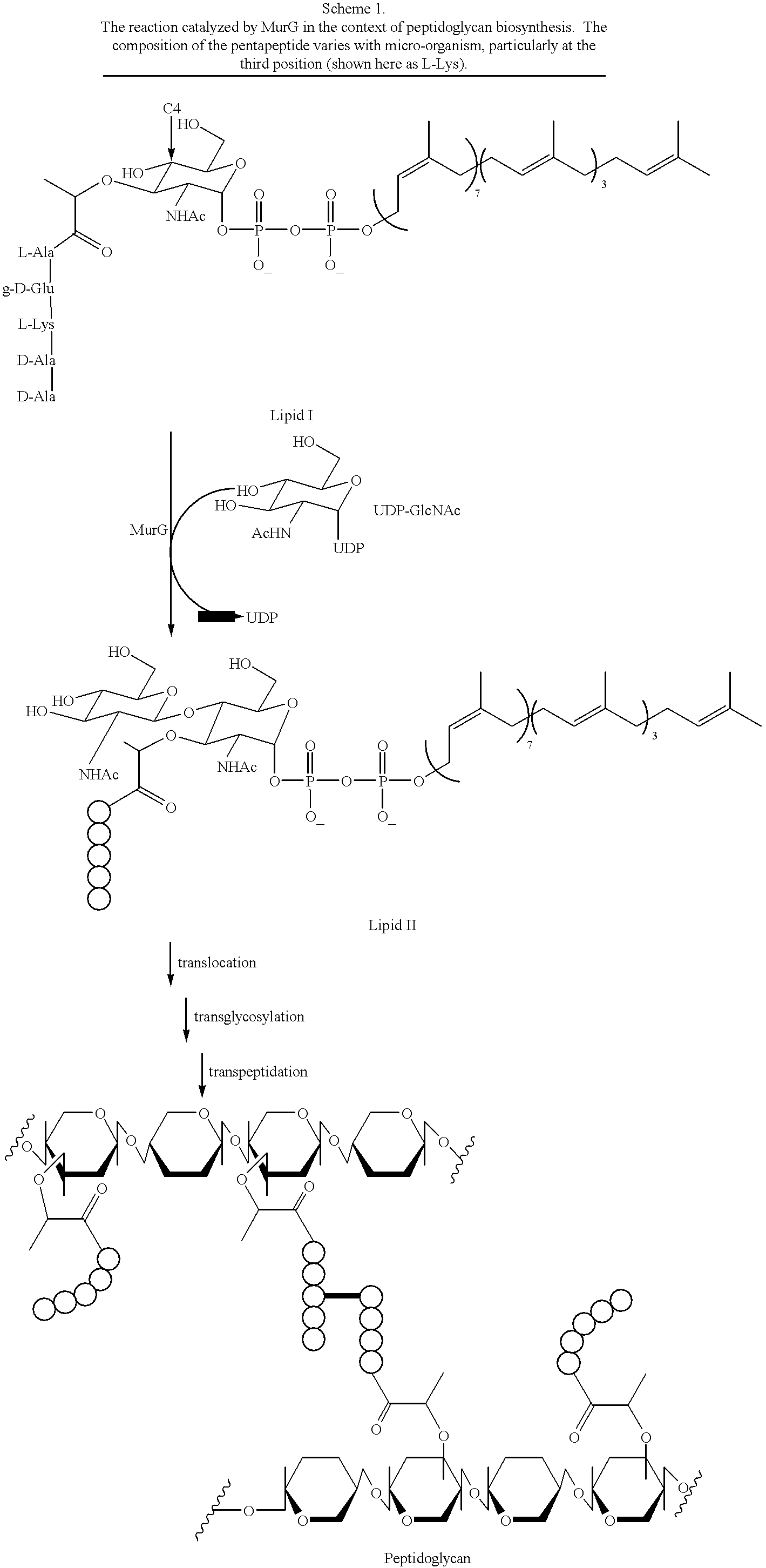
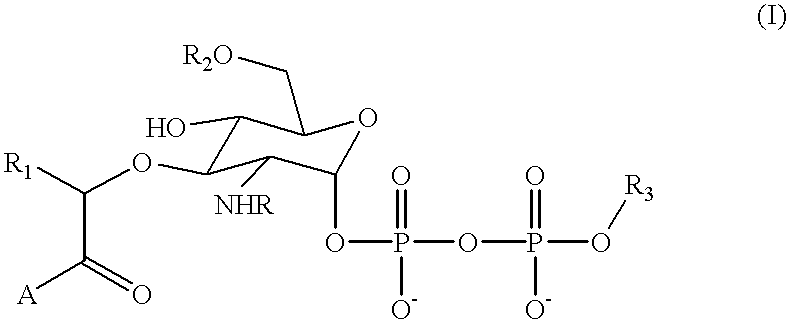
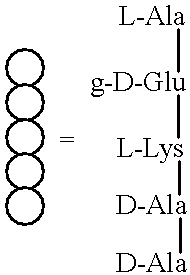Substrate analogs that substitute for lipid I as a substrate for MurG
a technology of murg and substrate, which is applied in the direction of esterified saccharide compounds, peptide/protein ingredients, transferases, etc., can solve the problems of inability to readily obtain or store substrates for and the study of most of the downstream enzymes is difficult. to achieve the effect of facilitating the separation of labeled udp-glcna
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
5.1. General Aspects of the Invention
The present invention contemplates a substance comprising the chemical moiety of the formula: ##STR4##
in which "R" is an acyl group comprising 2 or more carbon atoms, "R.sub.1 " is a substituted or unsubstituted alkyl group comprising 1 or more carbon atoms, "R.sub.2 " is a hydrogen or a substituted or unsubstituted alkyl group comprising 1 or more carbon atoms, "A" is a substituted or unsubstituted amino acid residue or a peptide comprising 2 or more substituted or unsubstituted amino acid residues and "R.sub.3 " is a substituted or unsubstituted alkyl group comprising 5 or more carbon atoms, such as 15 to 40 carbon atoms (see 5.3) or 10 to 40 carbon atoms based on citronellol containing 10 carbon atoms. Preferably, the substance of the invention (sometimes referred to herein as a substrate analog or, simply, compound) exhibits a binding affinity for at least wild type MurG enzyme. More preferably, the substance of the invention serves as an acc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com