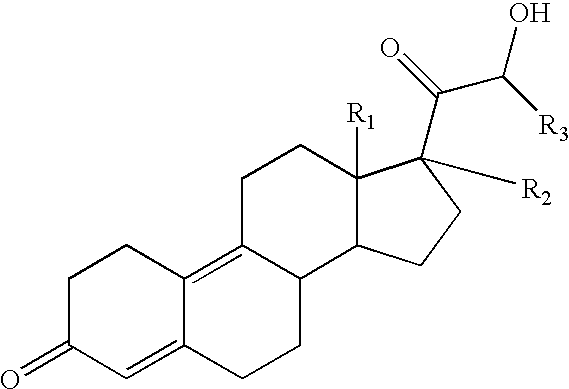
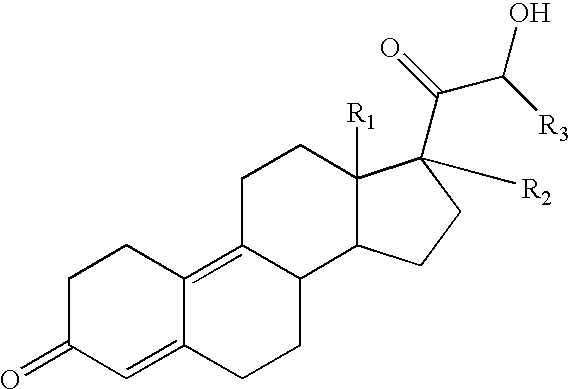
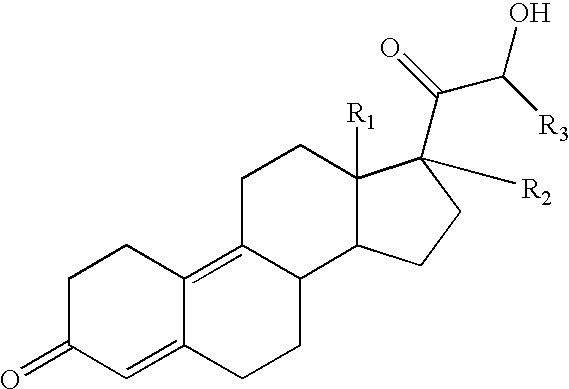
Use of estrogens and delta-gonadien-21-Ol-3,20-diones for treating insulin dependent and non-insulin dependent diabetes
a technology of estrogen and deltagonadien, which is applied in the direction of biocide, drug composition, metabolic disorder, etc., can solve the problems of diabetes, difficult control of this type of diabetes, and the most susceptible parts of the body,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
examples
Conjugated estrogens may be obtained following the process described in U.S. Pat. No. 2,720,483 or U.S. Pat. No. 2,565,115, which are incorporated herein by reference.
The compound having the formula II ##STR11##
may be prepared as described in U.S. Pat. No. 4,273,771. The compound of formula II is called trimegestone.
An example of a tablet contains a conjugated estrogen (0,6 mg) and a compound of formula II (2,4 mg) formulated with pharmaceutically acceptable carriers to provide a medicament for oral administration according to conventional methods. The formulation further include the following diluents, fillers, emulsifiers, preservatives, buffers and / or excipients, that is calcium phosphate tribasic, calcium sulfate, canauba wax, cellulose, glyceryl monooleate, lactose, magnesium stearate, methylcellulose, pharmaceutical glaze, polyethylen glycol, sucrose, povidone, titanium dioxide and red ferric oxide. One skilled in this art may formulate the tablet composition in an appropriate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| pharmaceutical compositions | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com