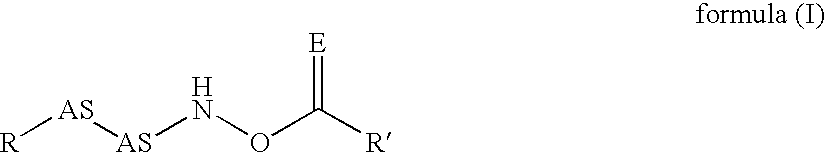
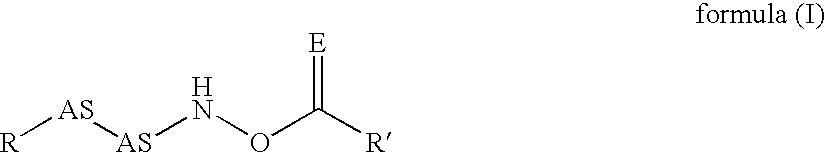
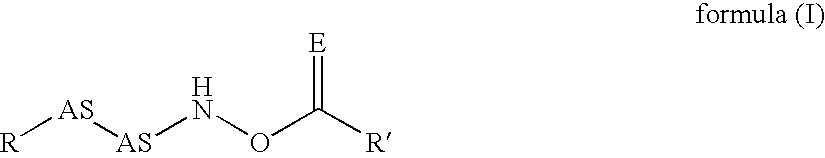
Inhibitors of dipeptidyl peptidase I
a technology of dipeptides and inhibitors, applied in the direction of peptide sources, peptide/protein ingredients, drug compositions, etc., can solve the problems of rapid intramolecular decomposition and n-terminal unprotected dipeptide derivatives, and achieve the effect of improving the wound healing process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of TFA*H-Gly-L-Phe-NHO-Ac (10)
TFA*H-Gly-L-Phe-NHO-Ac (10) was prepared according to Method D. The compound was purified by flash chromatography to give the product as a white solid (93%) of m.p. 71-73° C.—TLC (n-BuOH / ethyl acetate / water / acetic acid, 1:1:1:1): Rf=0.73.—1H NMR (400 MHz, DMSO-d6): δ=2.16 (s, 3H, CH3), 2.80 (dd, 1H, J=14.0 Hz, J=9.7 Hz, CH2 Phe), 3.04 (dd, 1H, J=13.9 Hz, J=9.6 Hz, CH2 Phe), 3.40 (d, 1H, J=16.3 Hz, CH2 Gly), 3.55 (d, 1H, J=16.3 Hz, CH2 Gly), 4.58-4.64 (m, 1H, CH Phe), 7.19-7.30 (m, 5H, aryl-H), 8.84 (d, 1H, J=8.4 Hz, NH).—MS (EI) m / z (%): 280 [M+H+].
example 2
Synthesis of TFA*H-Gly-L-Phe-NHO-Bz (11)
TFA*H-Gly-L-Phe-NHO-Bz (11) was prepared according to Method D. The compound was purified by flash chromatography to give the product as a white solid (66%) of m.p. 75-80° C.—TLC (n-BuOH / ethyl acetate / water / acetic acid, 1:1:1:1): Rf=0.81.—1H NMR (500 MHz, DMSO-d6): δ=2.86 (dd, 1H, J=13.9 Hz, J=10.0 Hz, CH2 Phe), 3.13 (dd, 1H, J=13.9 Hz, J=9.9 Hz, CH2 Phe), 3.43 (d, 1H, J=16.3 Hz, CH2 Gly), 3.58 (d, 1H, J=16.3 Hz, CH2 Gly), 4.68-4.72 (m, 1H, CH Phe), 7.21-7.25 (m, 1H, aryl-H), 7.26-7.31 (m, 4H, aryl-H), 7.58-7.63 (m, 2H, aryl-H), 7.74-7.77 (m, 1H, aryl-H), 7.93-8.03 (m, 2H, aryl-H), 7.94 (s, br., 3H, NH3+), 8.91 (d, 1H, J=8.3 Hz, NH), 12.46 (s, br., 1H, NH).—MS (EI) m / z (%): 342 [M+H+].
example 3
Synthesis of TFA*H-Gly-L-Phe-NHO-Bz-p-CH3 (12)
TFA*H-Gly-L-Phe-NHO-Bz-p-CH3 (12) was prepared according to Method D. The compound was purified by flash chromatography to give the product as a white solid (82%) of m.p. 98-101° C.—TLC (n-BuOH / ethyl acetate / water / acetic acid, 1:1:1:1): Rf=0.75.—1H NMR (400 MHz, DMSO-d6): δ=2.41 (s, 3H, CH3), 2.85 (dd, 1H, J=13.7 Hz, J=10.0 Hz, CH2 Phe), 3.12 (dd, 1H, J=12.9 Hz, J=10.0 Hz, CH2 Phe), 3.42 (d, 1H, J=15.8 Hz, CH2 Gly), 3.58 (d, 1H, J=16.0 Hz, CH2 Gly), 4.66-4.72 (m, 1H, CH Phe), 7.21-7.25 (m, 1H, aryl-H), 7.27-7.31 (m, 4H, aryl-H), 7.40 (d, 2H, J=8.0 Hz, aryl-H), 7.90 (d, 2H, J=8.2 Hz, aryl-H), 7.94 (s, br., 3H, NH3+), 8.90 (d, 1H, J=8.4 Hz, NH), 12.40 (s, br., 1H, NH).—MS (EI) m / z (%): 356 [M+H+].
PUM
| Property | Measurement | Unit |
|---|---|---|
| chemically stable | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| reactive affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com