Voxel-based approach for disease detection and evolution
a disease detection and evolution technology, applied in the field of systems and methods for monitoring tissue regions, can solve the problem that contrast changes in images taken over time may be difficult to detect by traditional qualitative visual assessmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
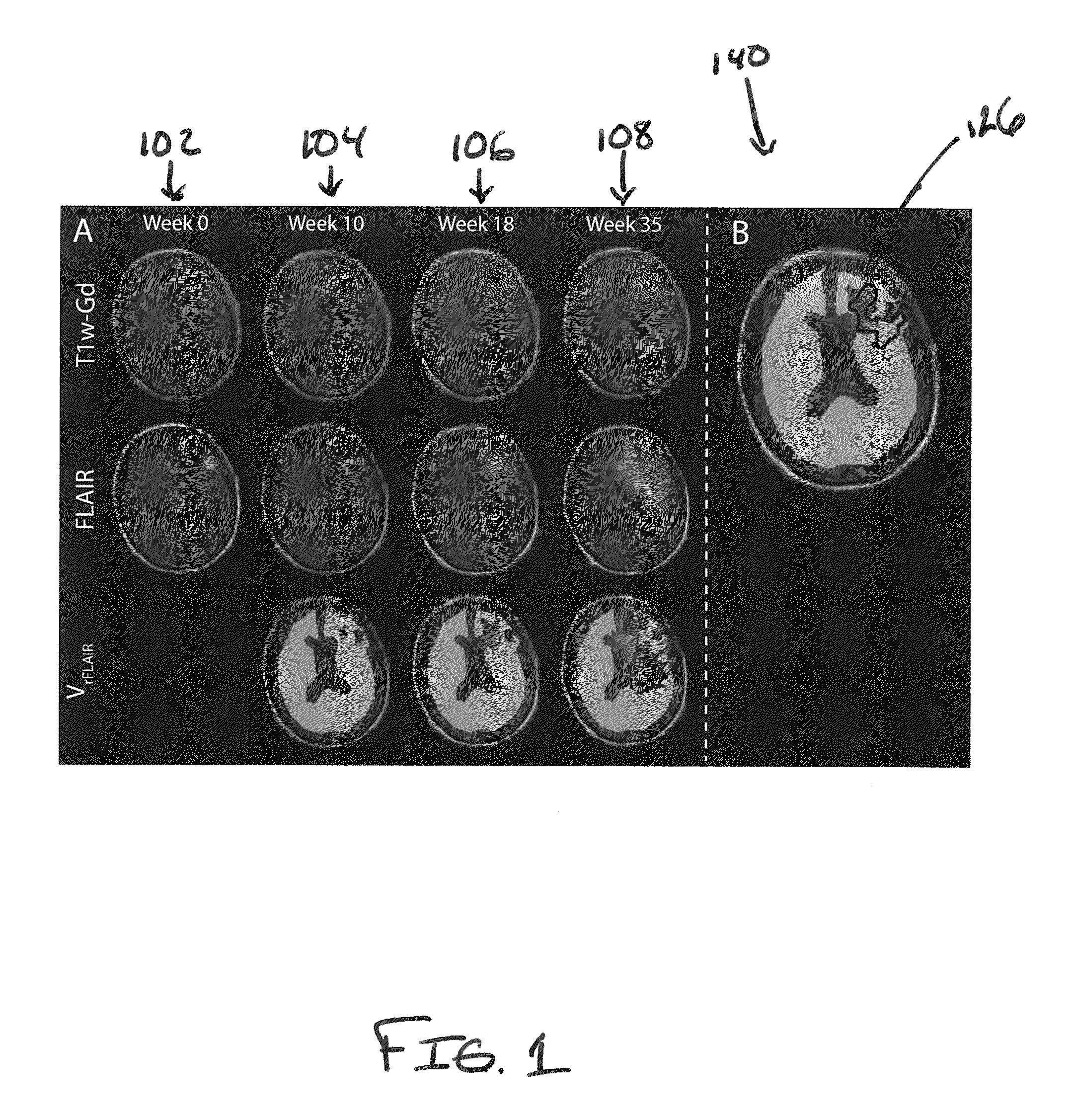
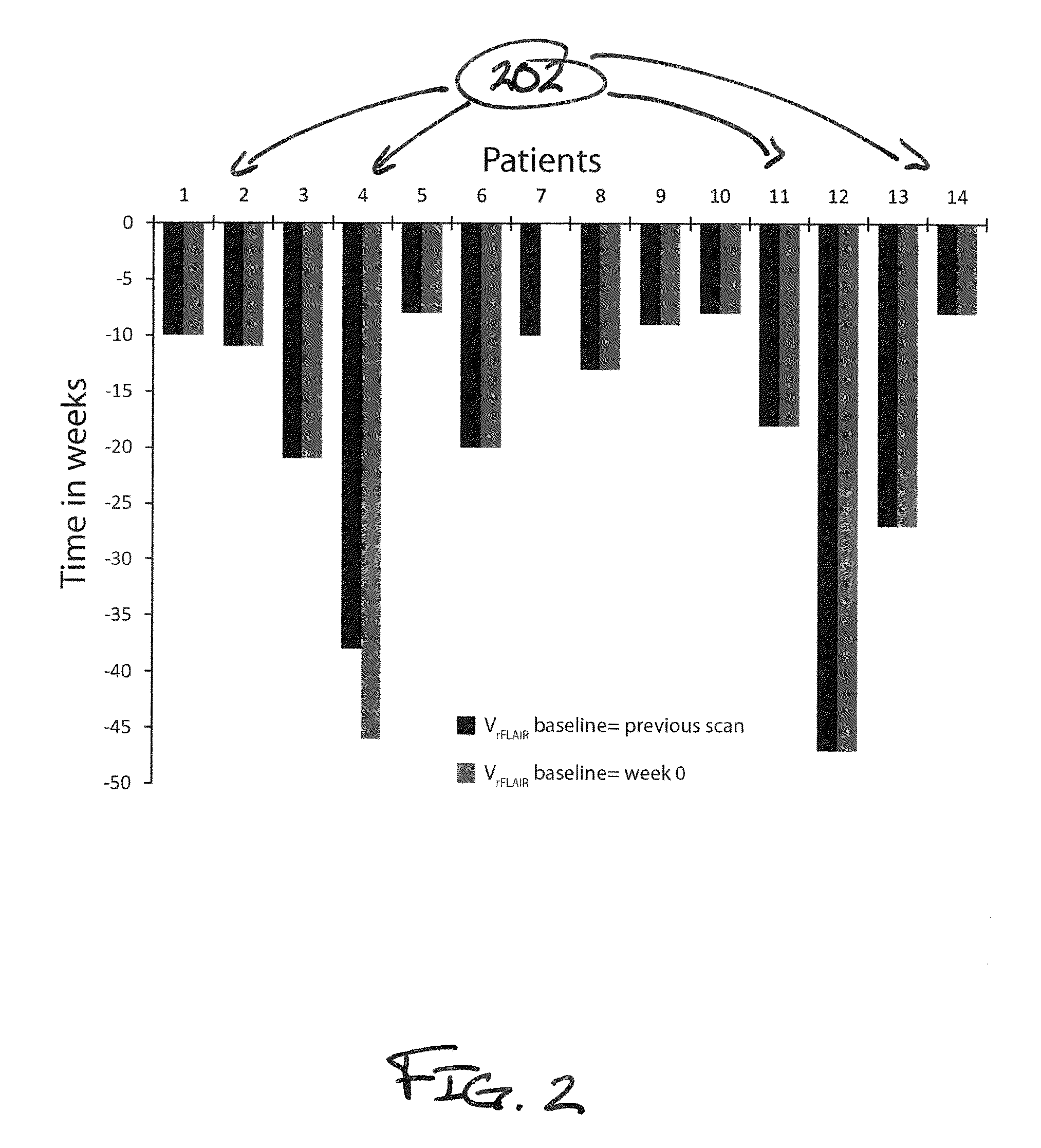
[0031]Methods of the present disclosure were demonstrated in a cohort of 14 glioma patients and compared to standard MRI-based criteria of clinical progression. The fourteen patients with pathologically proven grade III / IV gliomas were enrolled on a protocol of intra-treatment MRI. MRI scans were performed before and during treatment (every 2 months) until tumor recurrence was identified by Macdonald Criteria. All images were acquired on 1.5T or 3T MRI scanners. The MR1 protocol included fluid-attenuated inversion recovery imaging (FLAIR) and contrast-enhanced (Gd-DTPA) T1-weighted imaging. To avoid variability between scanners, subjects used the original scanner for all subsequent scans.
[0032]Subsequent to voxel-based analysis, FLAIR images were normalized to the mean signal intensity of white matter tracks (rFLAIR). All image data was registered to pre-treatment Gd-enhanced T1-weighted images using mutual information as an objective function and Nelder-Mead simplex as an optimizer...
example pcm
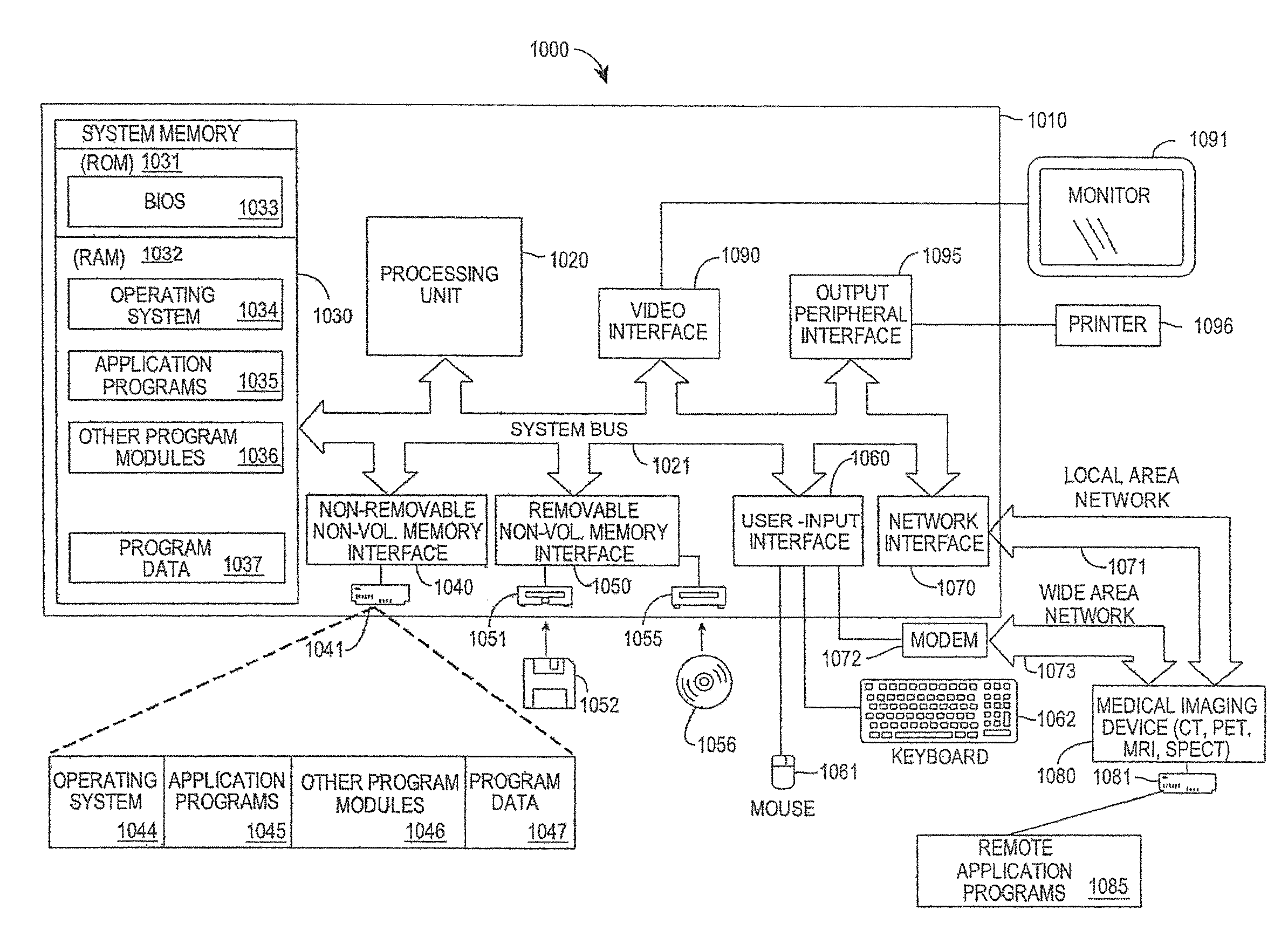
[0038]FIG. 3 is a block diagram of an example computer system 1000 on which a PRM system may operate, in accordance with the described embodiments. The computer system 1000 may be a PRM system, for example. The computer system 1000 includes a computing device in the form of a computer 1010 that may include, but is not limited to, a processing unit 1020, a system memory 1030, and a system bus 1021 that couples various system components including the system memory to the processing unit 1020. The system bus 1021 may be any of several types of bus structures including a memory bus or memory controller, a peripheral bus, and a local bus using any of a variety of bus architectures. By way of example, and not limitation, such architectures include the Industry Standard Architecture (ISA) bus, Micro Channel Architecture (MCA) bus, Enhanced ISA (EISA) bus, Video Electronics Standards Association (VESA) local bus, and Peripheral Component Interconnect (Pa) bus (also known as Mezzanine ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com