Stable liquid formulation of AMG 416 (etelcalcetide)
a liquid formulation and stable technology, applied in the field of liquid formulation, can solve the problems of disulfide bonds, peptides in general, and peptides containing disulfide bonds, which have only moderate or poor stability in aqueous solution, and can be unstabl
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Solubility of AMG 416 in Succinate Buffered Saline
[0087]In this study, the solubility of AMG 416 in succinate buffered-saline was investigated. AMG 416 HCl (103 mg powder, 80 mg peptide) was dissolved in 200 μL of sodium succinate buffered saline (25 mM succinate, 0.9% saline, pH 4.5). After briefly vortexing, a clear solution was obtained with a nominal concentration of 400 mg / mL. Because expansion of the solution volume was not determined, the solubility of AMG 416 can be conservatively stated as at least 200 mg / mL. Although the maximal solubility was not determined in this experiment, AMG 416 is soluble in pH 4.5 succinate buffered saline to concentrations of at least 200 mg / mL.
example 2
Concentration Dependent Stability Study
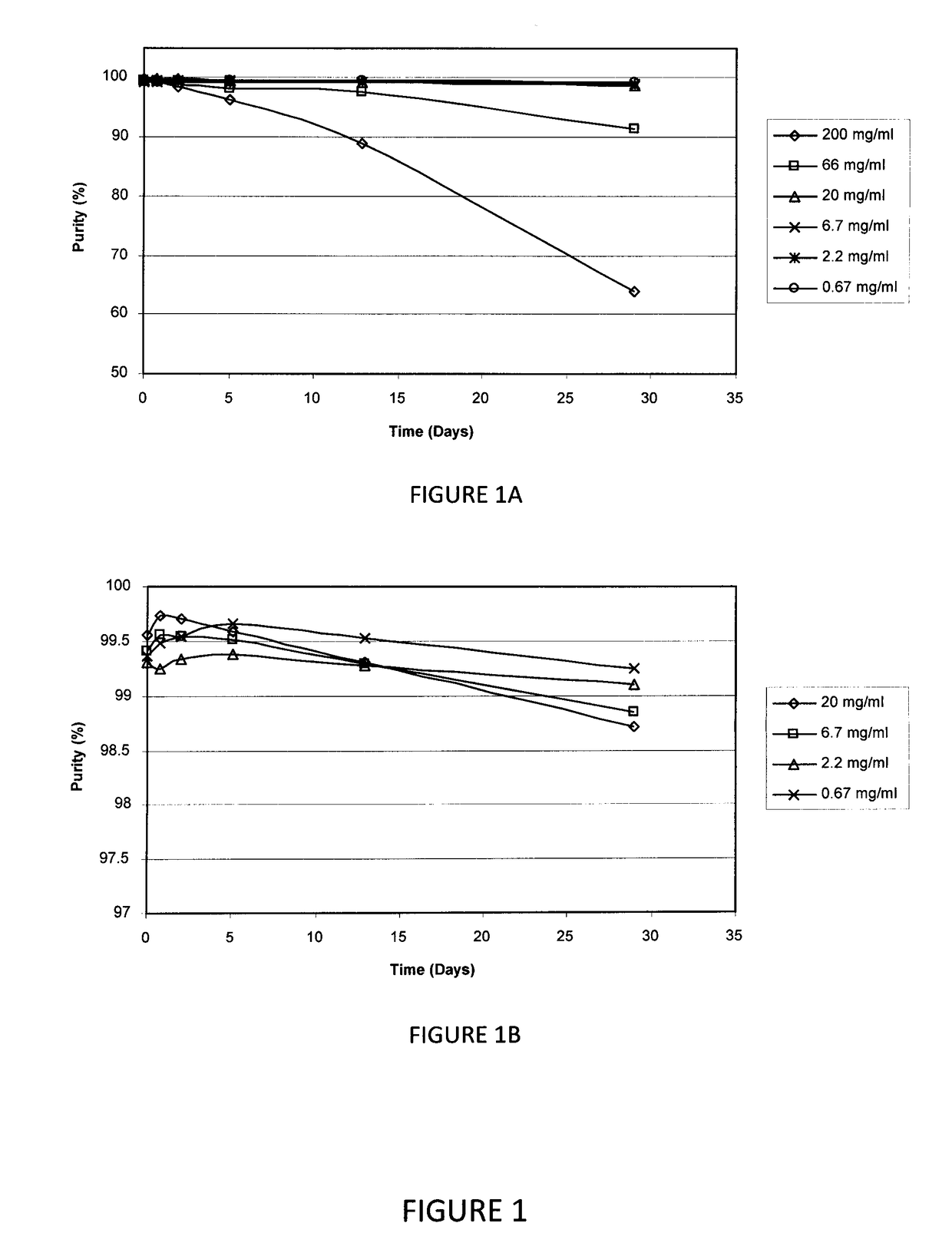
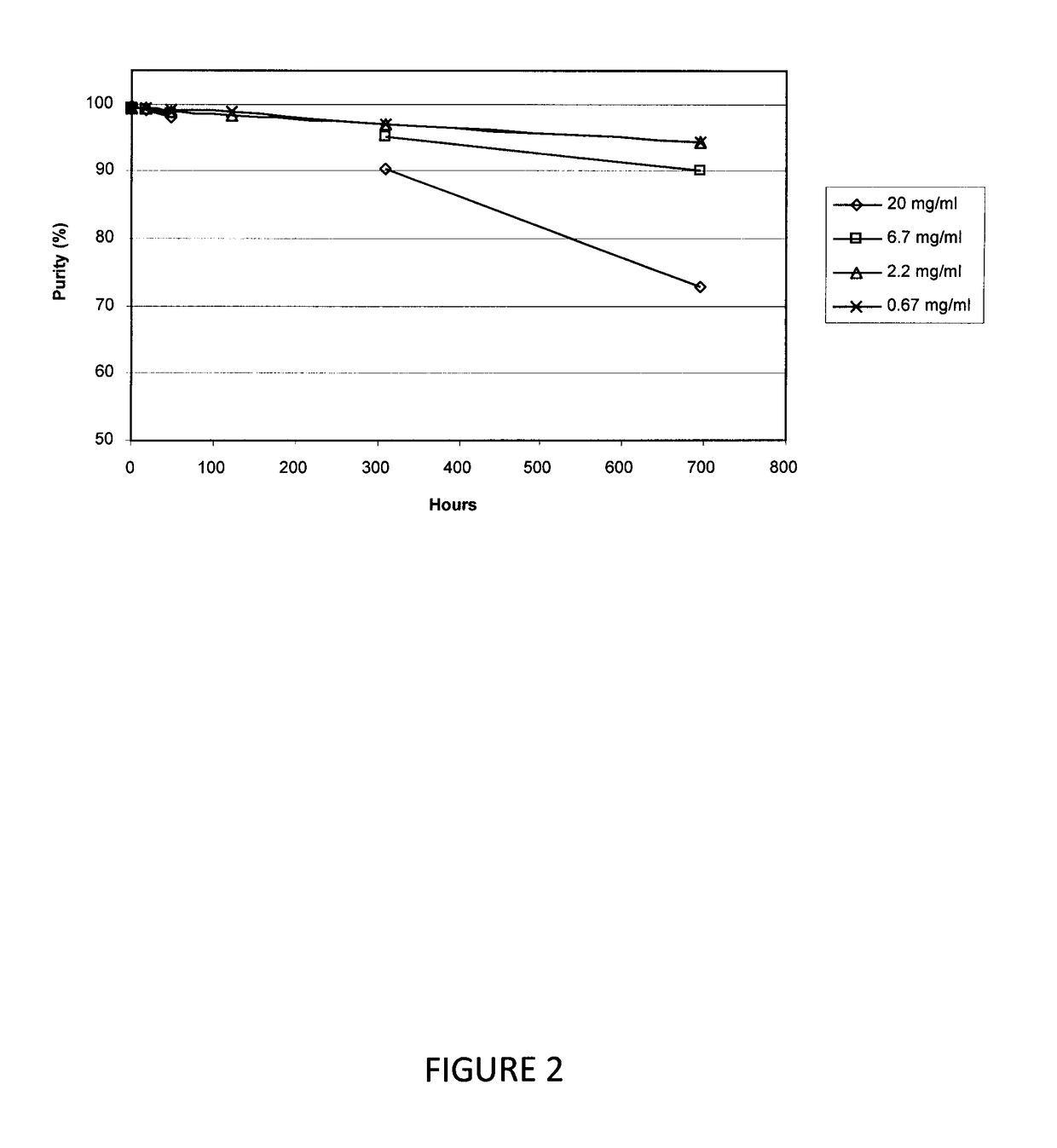
[0088]In this study, the stability of AMG 416 over a range of concentrations in succinate-buffered saline (pH 4.5) was investigated. The solution of 200 mg / mL AMG 416 from Example 1, supra, was diluted further with 200 μL of succinate-buffered saline (pH 4.5) to a nominal concentration of 200 mg / mL, which was serially diluted with succinate-buffered saline (pH 4.5) to 66, 20, 6.7, 2.2 and 0.67 mg / mL. The samples were kept at room temperature (i.e., about 25° C.) and aliquots were analyzed by HPLC at intervals up to 29 days. A second series of AMG 416 samples covering the concentration range 20 to 0.67 mg / mL were incubated at 40° C. and analyzed in the same manner.
[0089]The purity at the 29-day time point for samples at room temperature and 40° C. is provided in Tables 1 and 2, respectively. The results provide a stability profile of AMG 416 as a function of concentration and temperature.
[0090]
TABLE 1RT Stability of AMG 416 in 25 mM succinate-bu...
example 3
Stability of Liquid Formulations of AMG 416 Over Range of pH
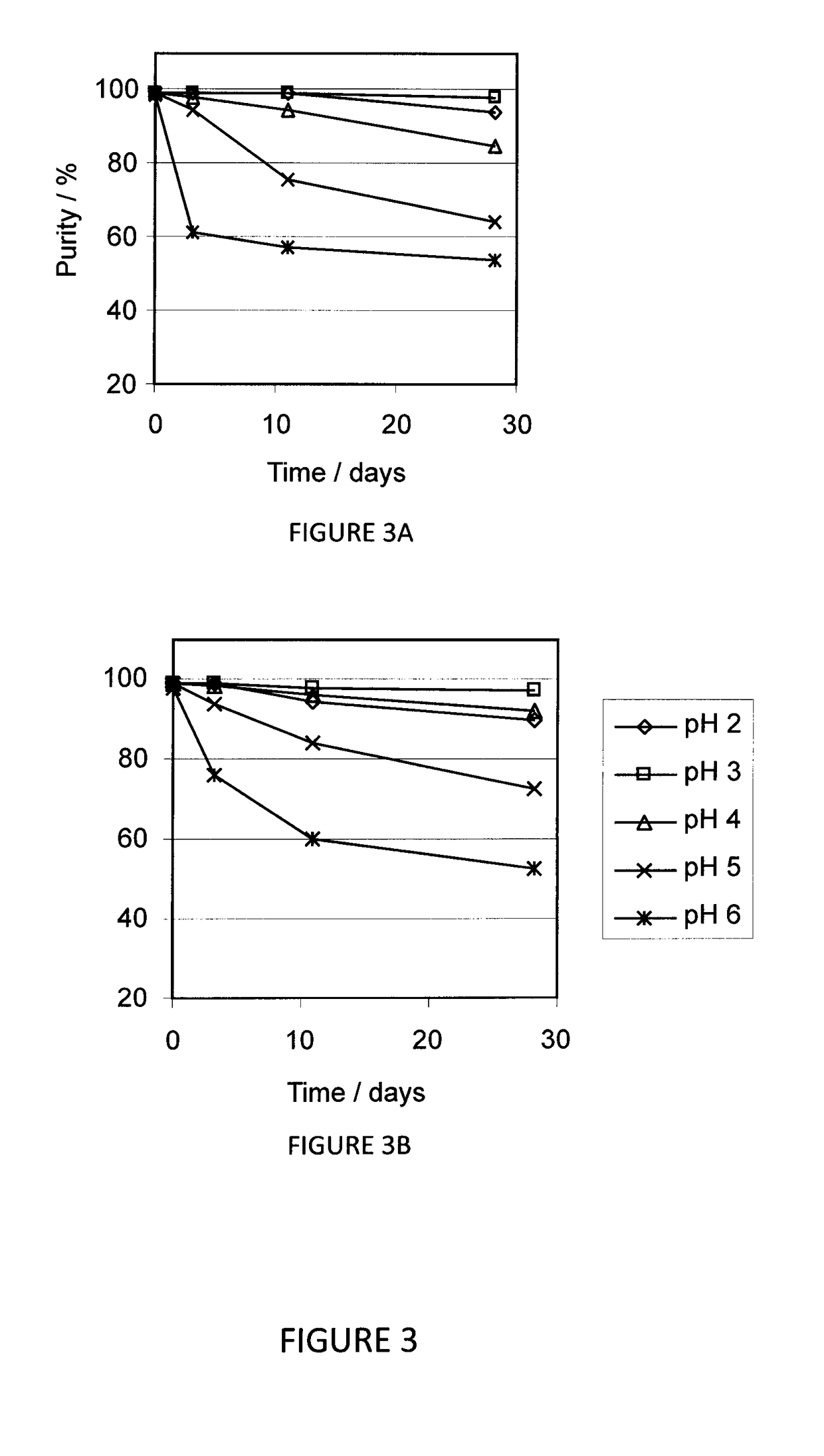
[0098]In this study, the stability of liquid formulations of AMG 416, at a concentration of 10 mg / mL, was determined over a range of pH in succinate-buffered saline. AMG 416 HCl (257 mg powder) was dissolved in 20 ml of pH 4.5 succinate buffered saline to provide 10.0 mg / ml peptide concentration (adjusted for peptide content of powder). The solution was divided evenly into five 4 mL portions which were adjusted to pH 2, 3, 4, 5 and 6, respectively, with NaOH and HCl as needed. Three 1 mL solutions were aliquoted from each portion and incubated at 2-8° C., room temperature (about 25° C.), and 40° C., respectively. The remaining 1 mL solution in each aliquot was diluted with pH 4.5 succinate buffered saline to 4 mL of 2.5 mg / mL peptide concentration, pH adjusted, and incubated in the same manner. Samples were retrieved according to schedule and diluted with deionized water to 1.0 mg / mL for HPLC analysis.
[0099]The purity at th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com