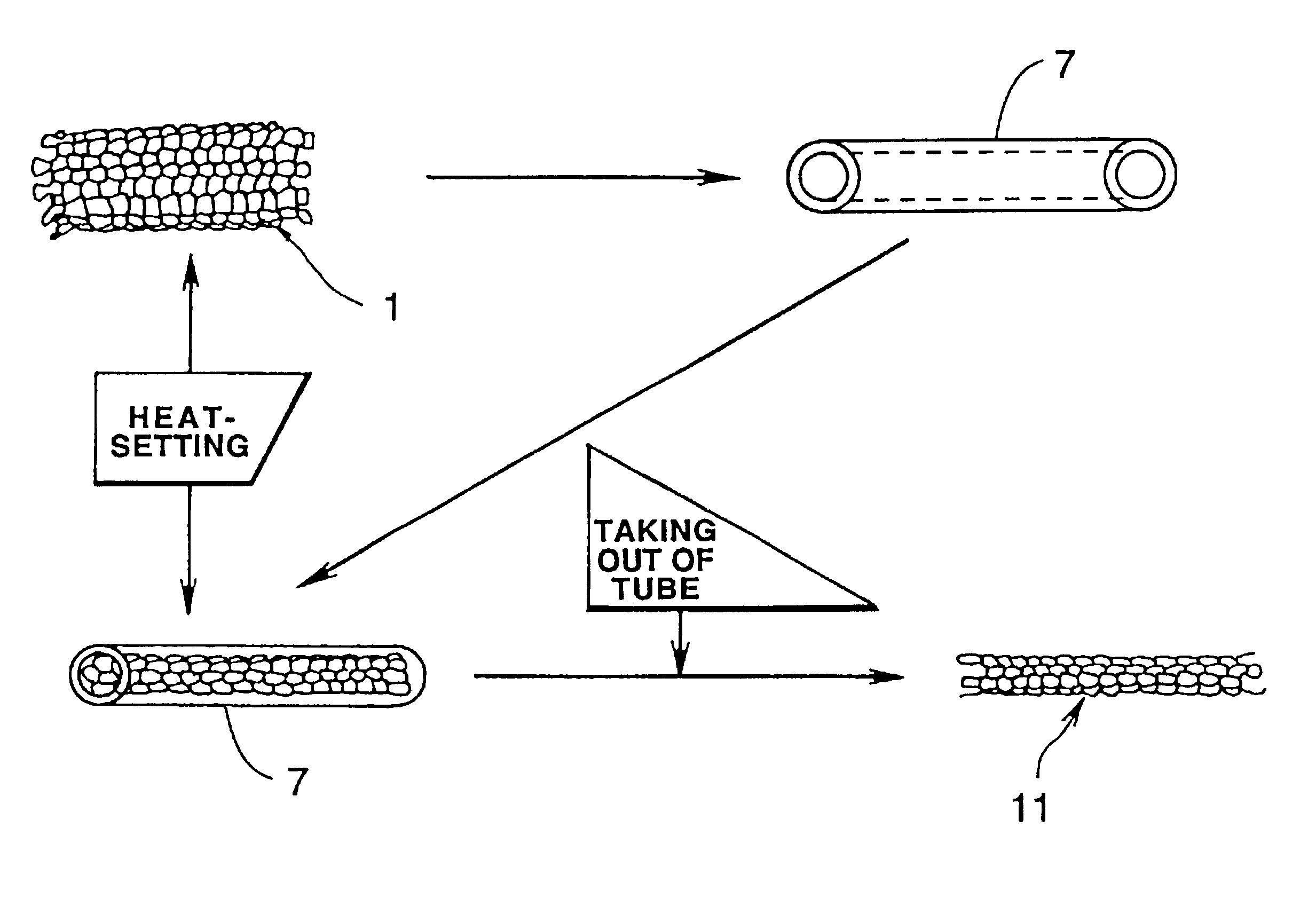
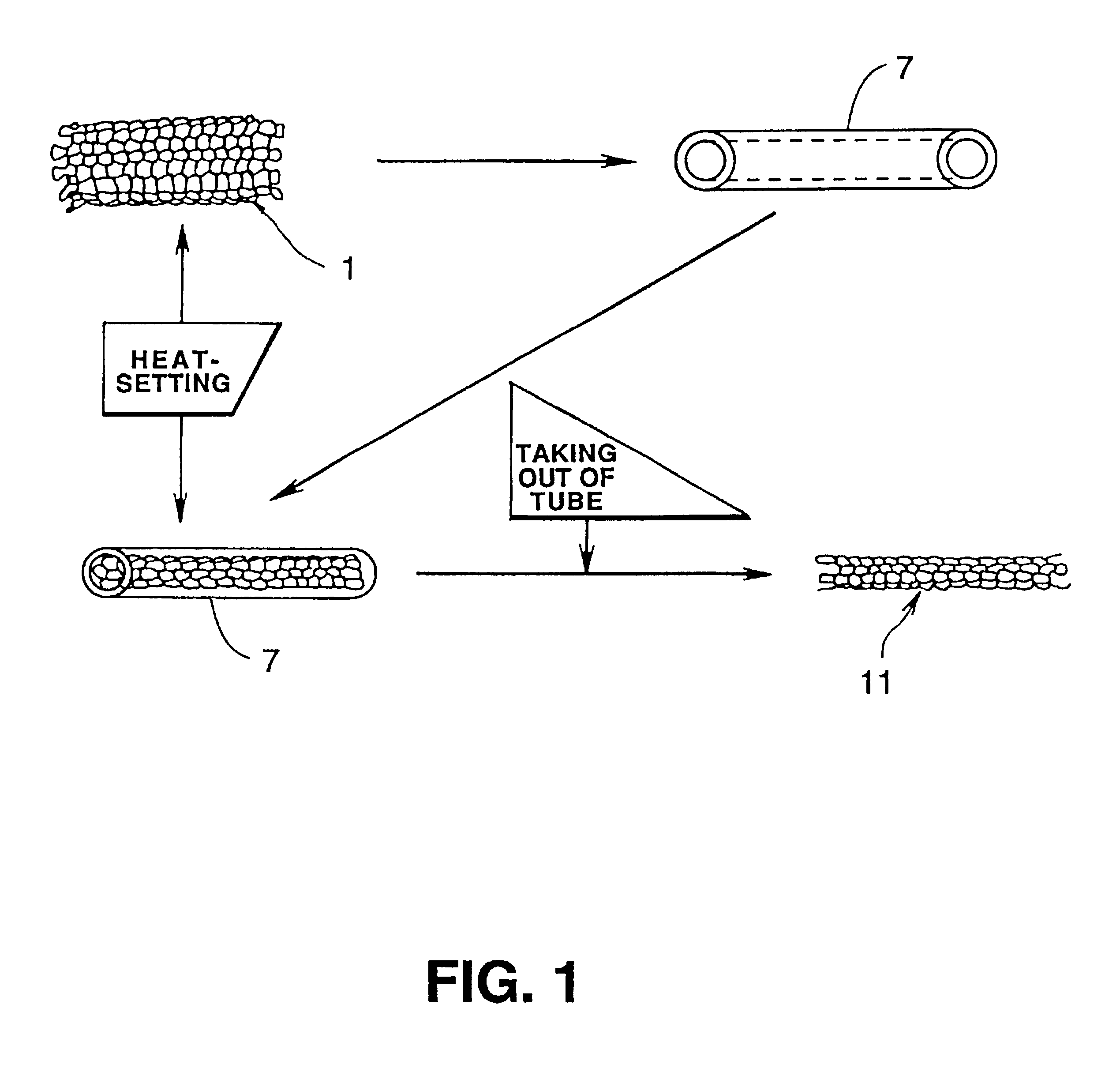
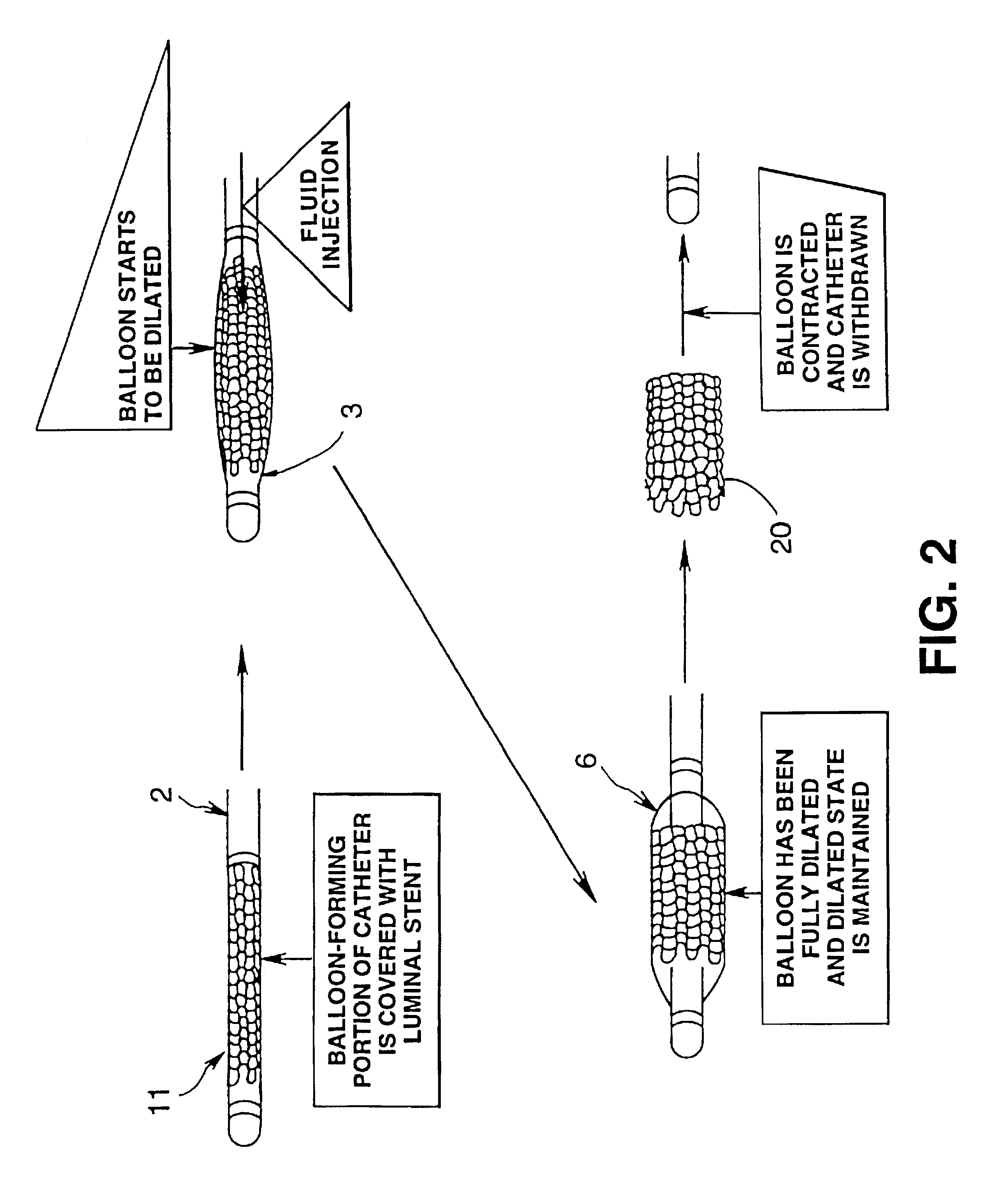
Luminal stent, holding structure therefor and device for attaching luminal stent
a technology of luminal stents and stents, which is applied in the field of stent introduction, can solve the problems of abnormal multiplication of living cells on the inner wall of the vessel to contract the vessel, high risk, and stenosis at the site of stent attachment,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment 1
Plural luminal stents formed by knitting a yarn of polylactic acid fibers admixed with barium sulfate were introduced and attached in the coronary of a test animal in a tubular state of 4 mm in diameter and 20 mm in length by using a catheter fitted with a balloon, and the state of attachment was observed by irradiation of X-rays. It was seen that the stents substantially maintained their shape until after about three to six months. It was seen that the stents disappeared by being absorbed into living tissue in about 6 to 12 months. During this time, no abnormalities such as inflammation or hypertrophy of the intima of the blood vessel were observed.
experiment 2
Plural luminal stents formed by knitting a yarn of polyglycolic acid fibers admixed with barium sulfate were introduced and attached in the femoral artery of a test animal in a tubular state of 4 mm in diameter and 20 mm din length and the state of attachment was observed by irradiation of X-rays. It was seen that the stents substantially maintained their shape until after about two to three weeks and were absorbed into the living tissue in about two to three months. The shape retention period and the period of existence in the living body attained in Experiment 2 are thought to be more safe than the corresponding periods attained in Experiment 1. Meanwhile, no inflammation or hypertrophy of the intima of the blood vessel was observed during these periods.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Shape | aaaaa | aaaaa |
| Bioabsorbable | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com