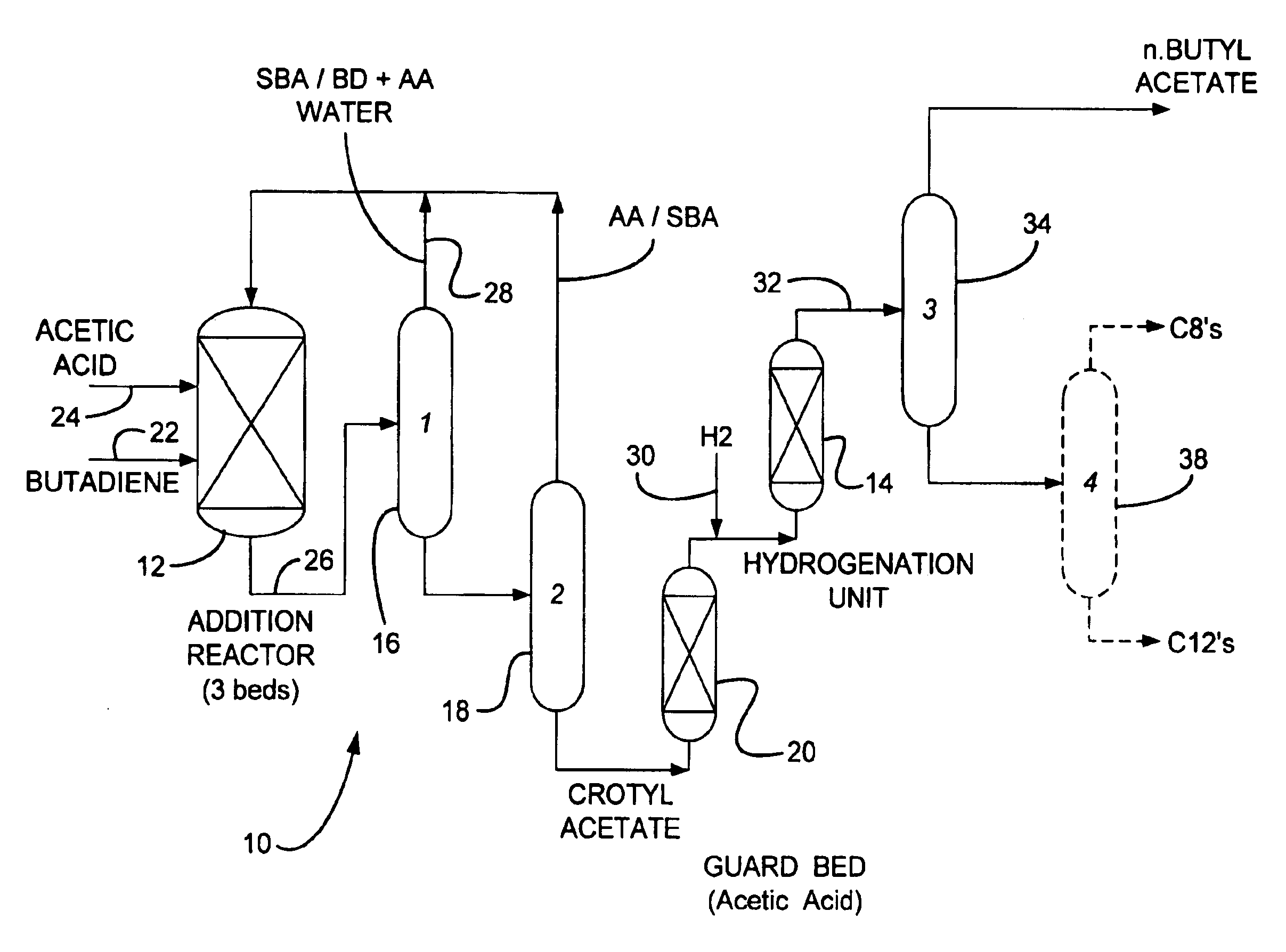
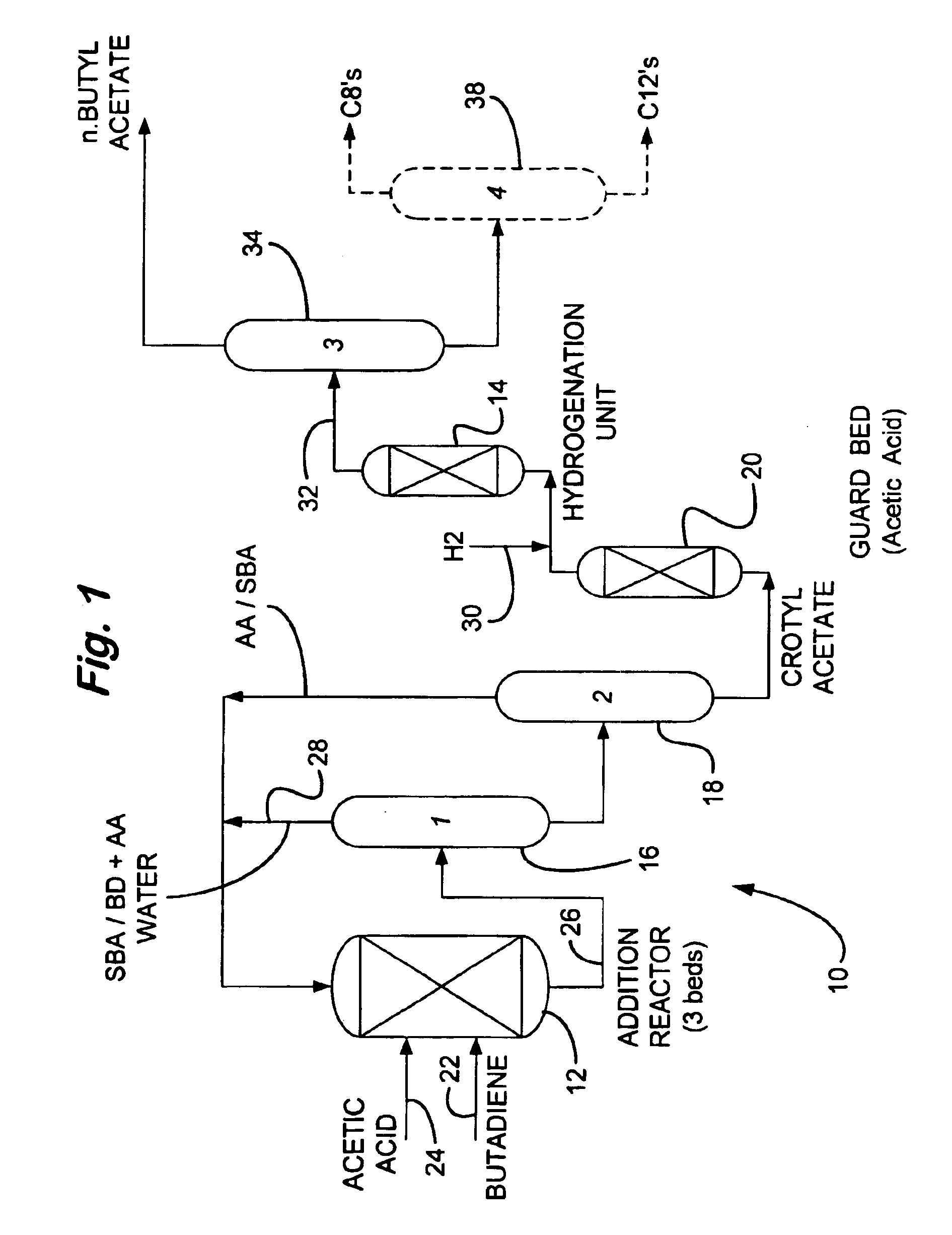
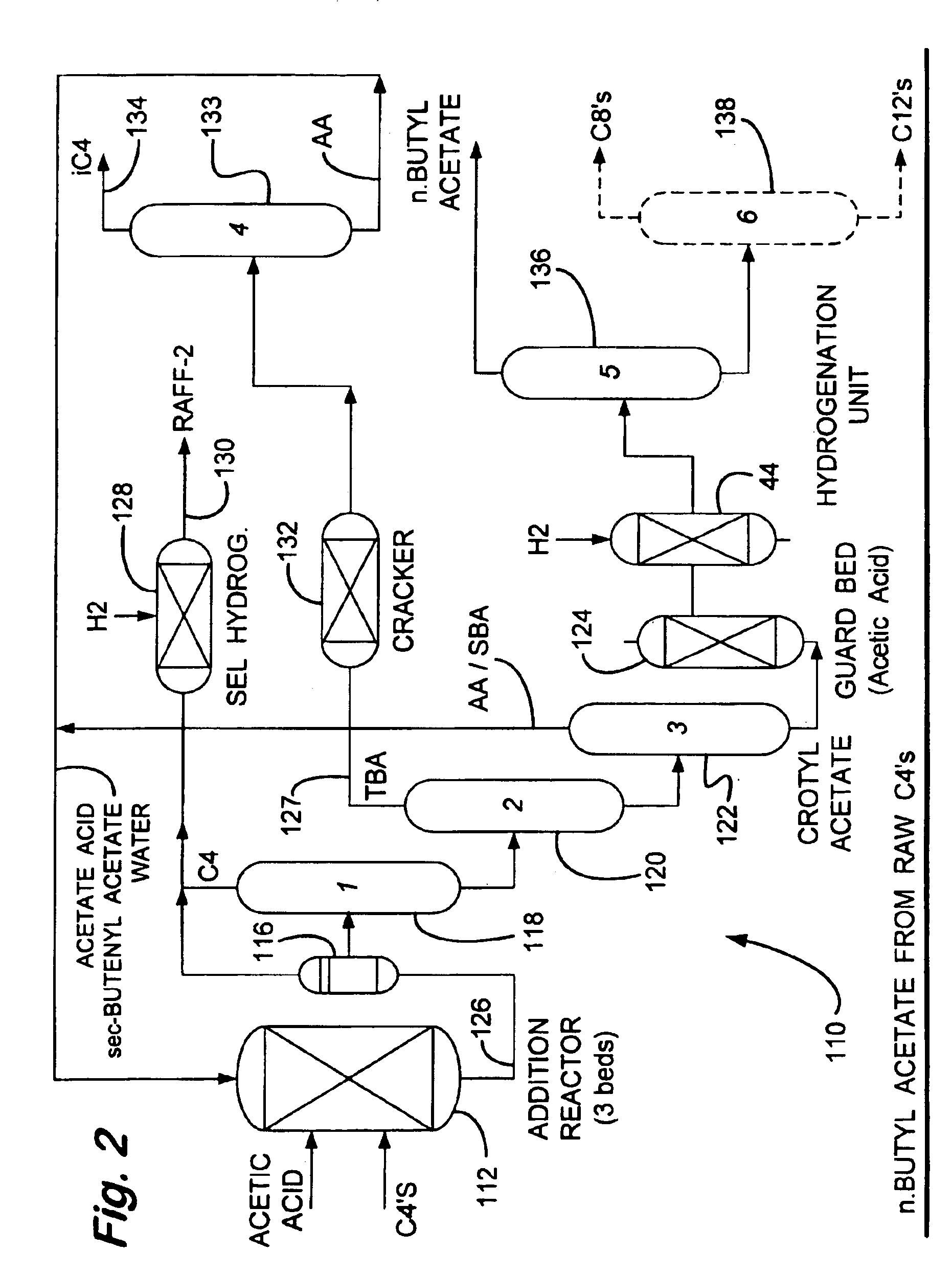
Process for making n-butyl esters from butadiene
a technology of butadiene and n-butyl esters, which is applied in the preparation of ester-hydroxy reactions, chemical apparatus and processes, organic chemistry, etc., can solve the problems of increasing capital costs, and achieve the effect of increasing activity and increasing the cost of reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0069]
sec-Butenyln-ButenylRuntimeacetateacetate4-Vinyl cyclohexeneHighers(Hours)(% w / w)(% w / w)(% w / w)(% w / w)0001.3057.77.591.32.8568.959.371.283.9179.4910.241.254.34810.4911.721.265.072410.9814.210.776.55
[0070]These results illustrate that the reaction proceeds to give predominantly the isomeric C4 acetates and that some loss of selectivity occurs to higher boiling point materials particularly at high reaction times. The reaction product was pale yellow which darkened on standing.
Example 2
Use of Amberlyst 15H® Resin with Pre-conditioning
[0071]The general method was used and the resin washed with ethyl acetate and dried prior to use. The following components were charged to the autoclave:[0072]Amberlyst 15H®—85 g[0073]Acetic acid—3600 g[0074]1,3-Butadiene—700 g[0075]Reaction conditions: 50° C., with stirring at 1200 rpm
example 2
[0076]
sec-Butenyln-ButenylRuntimeacetateacetate4-Vinyl cyclohexeneHighers(Minutes)(% w / w)(% w / w)(% w / w)(% w / w)0001.390301.080.721.310.21601.971.371.340.45902.832.021.350.711504.013.11.311.22105.34.371.261.752706.25.431.192.263306.966.361.192.713907.927.581.23.09
[0077]These results reaffirm the previous results and demonstrate that the sec-butenyl acetate is the kinetic reaction product and that the n-butenyl acetate is the thermodynamic product. The reaction product was initially colourless but darkened on standing to a pale yellow. This illustrates that pre-treatment of the Amberlyst 15H® resin served to reduce the colour of the product.
examples 3 and 4
Effect of Temperature on Reaction Rate
[0078]Two sequential reactions were carried out on the charge of 85 g of Amberlyst 15H® used in example 2 (ethyl acetate pre-washed), the catalyst between runs was washed with acetic acid in situ to remove residual material from the previous run in the sequential reactions. The charge of butadiene was 700 g and acetic acid was 3600 g for all three experiments.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com