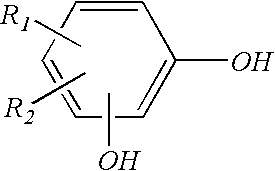
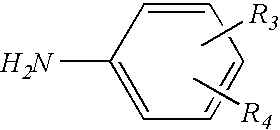
Resorcinolic derivative for rubber compounds
a technology of resorcinolic compounds and rubber, applied in the field of resorcinolic compounds for rubber reinforcement, can solve the problems of suggesting the use of 3-hydroxydiphenylamine as a rubber reinforcement compound, and achieve the effect of improving physical and mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0029]The following examples are intended to illustrate the invention and should not be construed as limiting the invention in any way.
example no.1
Example No. 1
[0030]Into a 500-ml round bottomed flask equipped with a mechanical stirrer, thermometer and Dean-Stark condenser was charged 0.5 mole (55.0 grams) of resorcinol, 1.5 mole (139.7 grams) of aniline and 2.2 grams of PTSA catalyst. The reaction mixture was heated to between 185-205° C.; about 11.0 grams of distillate was collected in the Dean-Stark trap in about 10.0 hours. After this, 1.0 gram of 50% wt. / wt. aqueous NaOH solution was added to neutralize the catalyst. Then, vacuum was applied to distill out the excess aniline under 28″ of Hg and 160-165° C. temperature conditions. The final reaction product weighed 91.9 grams and showed the following composition determined by LC / GC analysis.
[0031]
Composition (wt. %)Aniline5.7Resorcinol6.73-Hydroxydiphenylamine89.0Impurity (unknown)2.3
[0032]The melting point as determined by the capillary method was between about 65-70° C.
[0033]The above crude reaction product was recrystallized from an aqueous solution. The purified materi...
example no.2
Example No. 2
[0035]The procedure described in Example No. 1 was repeated with 1.5 moles of resorcinol, 2.25 moles of aniline and 6.0 grams of PTSA catalyst. Continuous passage of nitrogen gas was done to remove the water by-product formed during the reaction. The reaction took about 21.0 hours to remove 27.1 grams of water distillate. The product weighed 280.0 grams after distilling out the excess aniline. The melting point was determined to be between 68-72° C. The chemical composition determined by the LC / GC analysis showed the presence of aniline=2.6 wt. %, resorcinol=0.6 wt. % and unknown impurities=4.0 wt. %
[0036]Further purification of this crude material by an aqueous recrystallization method showed that the 3-hydroxydiphenylamine had a melting point of 71-75° C. and contained the following impurities (wt. %).
[0037]
Aniline0.07Resorcinol0.04Unknown4.6
PUM
| Property | Measurement | Unit |
|---|---|---|
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com