Multi-channel non-invasive tissue oximeter
a tissue oximeter and multi-channel technology, applied in the field of in vivo spectrophotometric examination, can solve the problems of difficult signal-to-noise problems, distortion of brain examination data, and/or obscure of data,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
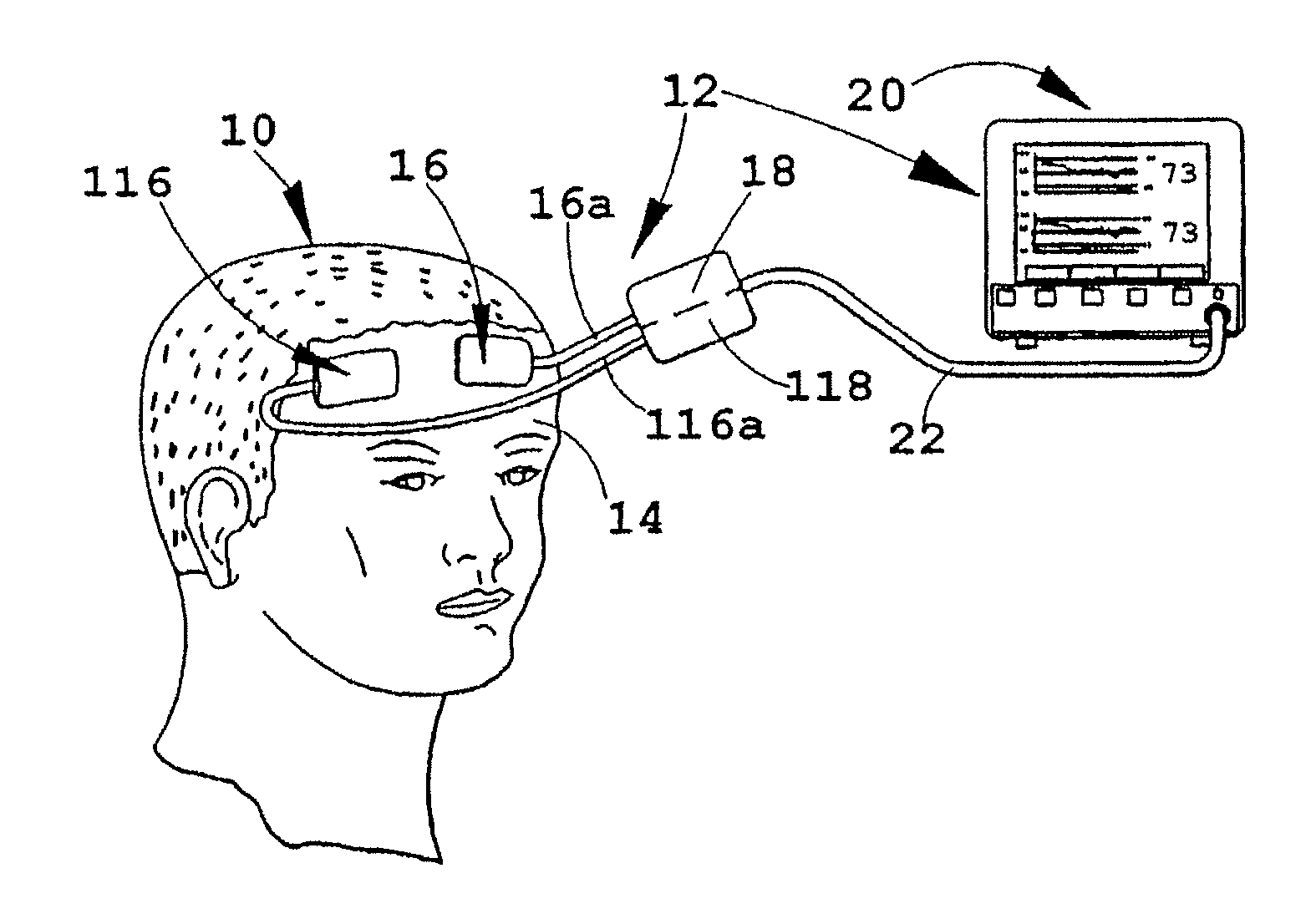
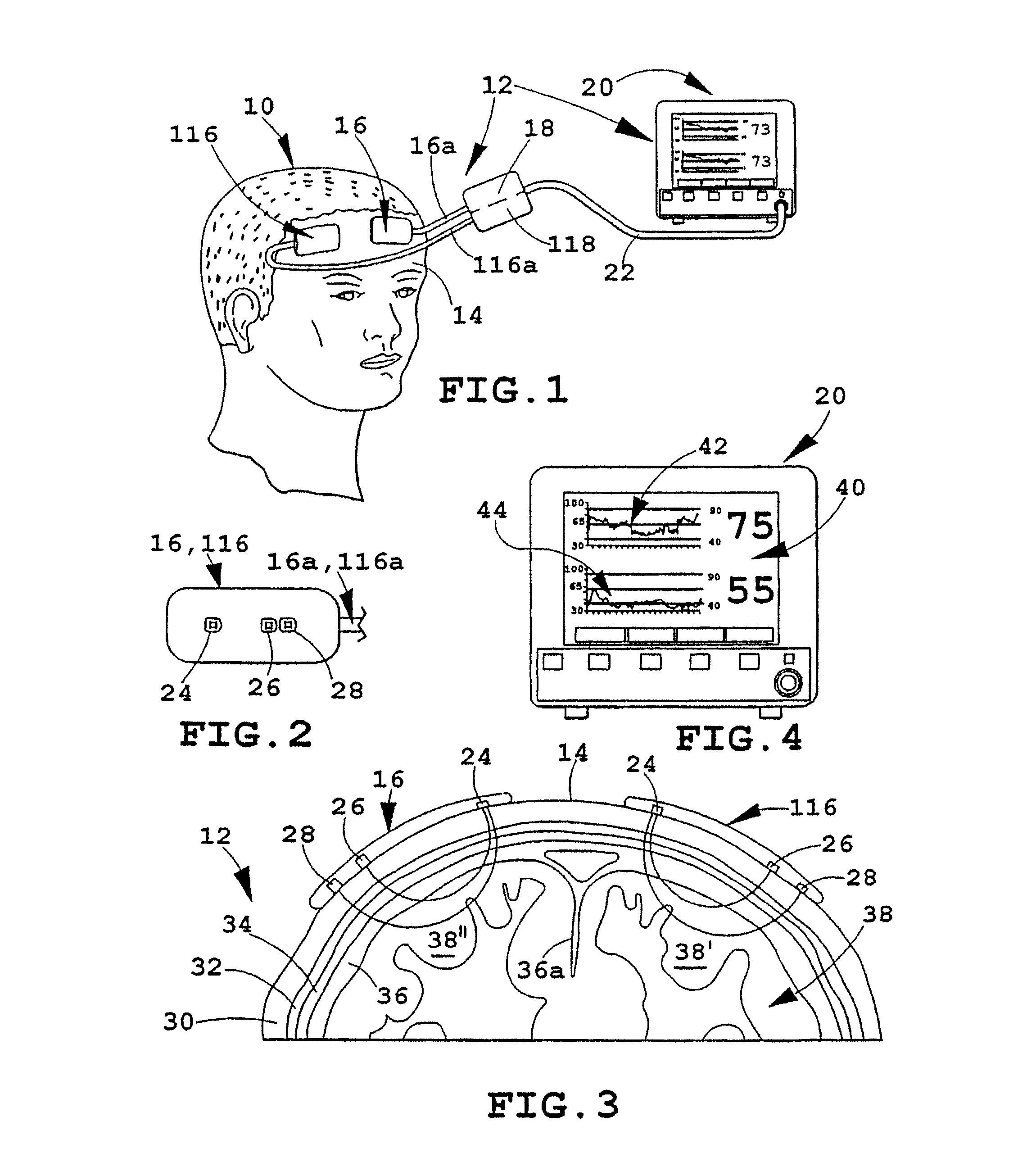
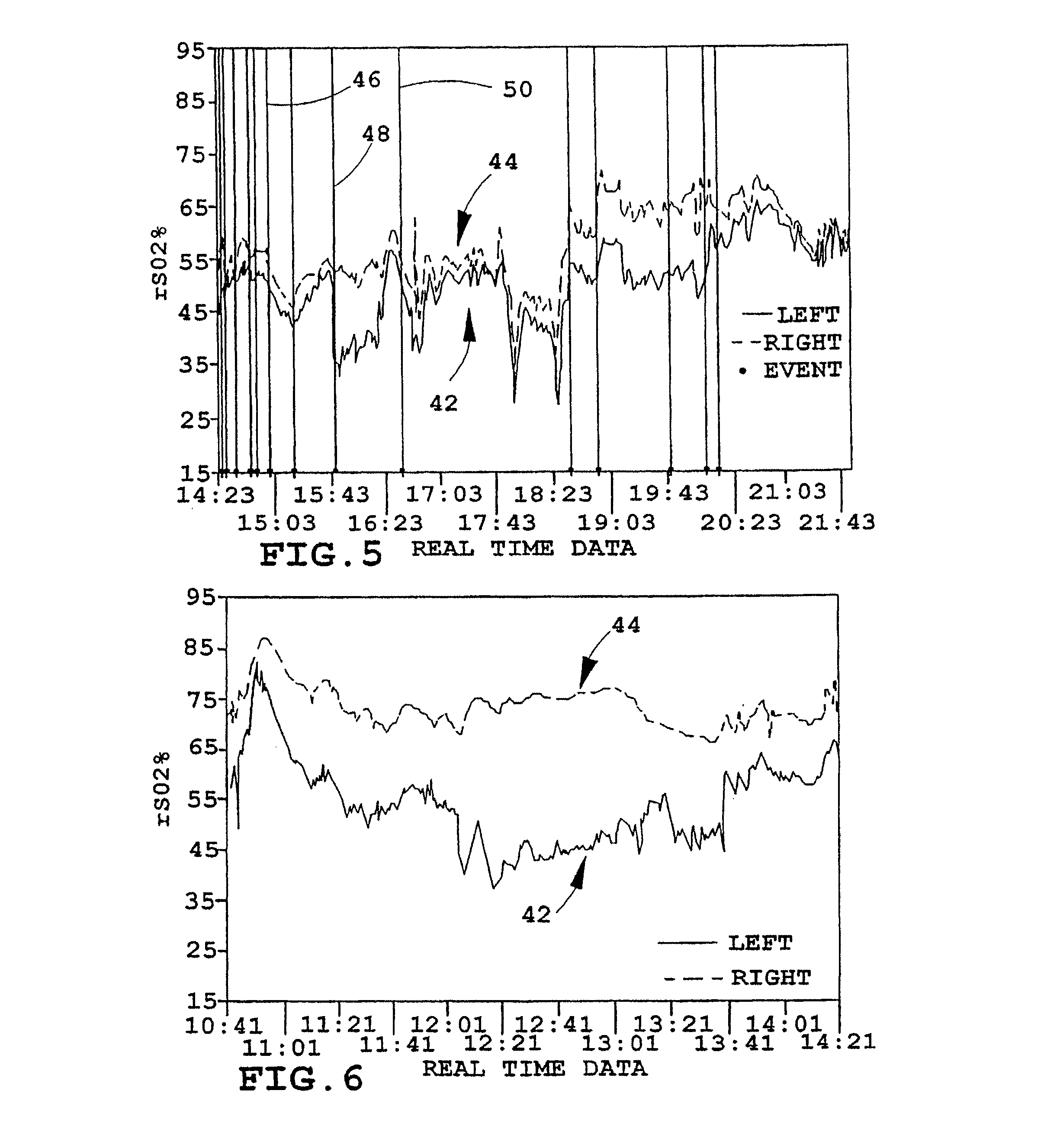
[0018]FIG. 1 depicts an illustrative patient 10 on whom an instrument 12 in accordance with the present invention is being employed. As illustrated, the forehead 14 of patient 10 has a pair of sensors 16, 116 secured to it in a bilateral configuration, i.e., one such sensor on each side of the forehead, where each may monitor a different brain hermisphere. Each of the sensors 16, 116 is connected to a processor and display unit 20 which provides a central control and processing station (sometimes hereinafter, referred to as the “oximeter”) by a corresponding electrical cable 16A, 116A, which join one another at a dual-channel coupler / pre-amp 18, 118 and then (preferably) proceed to the control and processor 20 as an integrated, multiple-conductor cable 22. As will be understood, the electrical cables just noted include individual conductors for energizing light emitters and operating the related light detectors contained in sensors 16, 116, all as referred to further hereinafter and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com