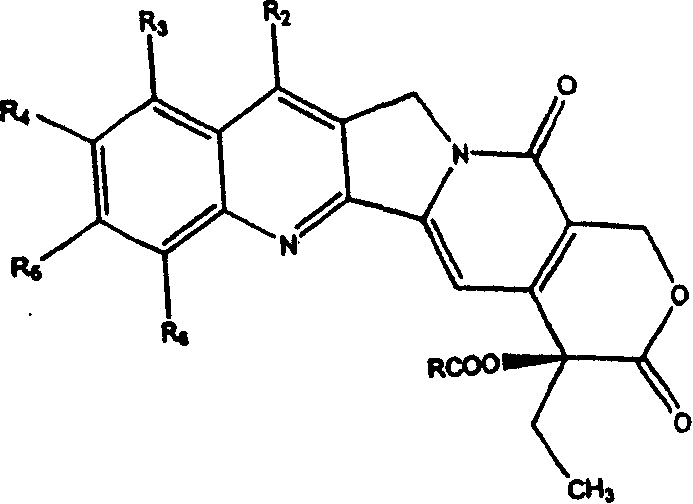
Camptothecin derivatives
一种化合物、低级的技术,应用在药物组合、杂环化合物有效成分、有机化学等方向,能够解决抗癌活性微乎其微、毒性等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0342] This example illustrates the preparation of camptothecin-20-O-esters of unsubstituted and substituted 4-fluorophenoxyacetic acid.
[0343] A. Camptothecin-20-O-ester of 4-fluorophenoxyacetic acid (000417)
[0344] A mixture of camptothecin (30mg, 0.086mmol), 4-fluorophenoxyacetic acid (30mg, 0.18mmol), EDCI (60mg, 0.31mmol), DMAP (5mg, 0.047mmol) and dichloromethane (5ml) was Stir at room temperature for 20 hours. Dichloromethane (20ml) was then added to the solution. The organic layer was washed with water (20ml), saturated NaHCO 3 Aqueous solution (10ml) and brine (20ml), then washed with MgSO 4 dry. After removing the solvent under reduced pressure, the resulting solid was separated by column chromatography (eluent: CHCl 3 :CH 3 OH 9:1), to obtain 33mg camptothecin-20-O-4-fluorophenoxy acetate, yield 76.7%, mp 227-229°C (decomposition).
[0345] Chemical structure analysis: 1 HNMR (CDCl 3 , 600MHz): δ8.41(s, 1H, Ar-H), 8.25(d, 1H, Ar-H), 7.96(d, 1H, Ar-H), 7...
Embodiment 2
[0377] This example illustrates the preparation of camptothecin-20-O-esters of unsubstituted and substituted 4-bromophenoxyacetic acid.
[0378] A. Camptothecin-20-O-ester of 4-bromophenoxyacetic acid (000315)
[0379] A mixture of camptothecin (30mg, 0.086mmol), 4-bromophenoxyacetic acid (41mg, mmol), EDCI (60mg, 0.31mmol), DMAP (5mg, 0.047mmol) and dichloromethane (5ml) was at room temperature Stirring was continued for 20 hours. Dichloromethane (20ml) was then added to the solution. The organic layer was washed with water (20ml), saturated NaHCO 3 Aqueous solution (10ml) and brine (20ml), then washed with MgSO 4 dry. After removing the solvent under reduced pressure, the resulting solid was recrystallized from ethyl acetate to give 42 mg of camptothecin-20-O-4-bromophenoxyacetate, yield 87.1%, mp 232-234°C (decomposition ).
[0380] Chemical structure analysis: 1 HNMR (CDCl3 , 600MHz): δ8.67(s, 1H, Ar-H), 8.26(d, 1H, Ar-H), 8.10(d, 1H, Ar-H), 7.90(t, 1H, Ar-H), 7.73...
Embodiment 3
[0412] This example illustrates the preparation of unsubstituted and substituted camptothecin-20-O-esters of 4-iodophenoxyacetic acid.
[0413] A. Camptothecin-20-O-ester of 4-iodophenoxyacetic acid (000413)
[0414] A mixture of camptothecin (30mg, 0.086mmol), 4-iodophenoxyacetic acid (36mg, 0.18mmol), EDCI (60mg, 0.31mmol), DMAP (5mg, 0.047mmol) and dichloromethane (5ml) was Stir at room temperature for 20 hours. Dichloromethane (20ml) was then added to the solution. The organic layer was washed with water (20ml), saturated NaHCO 3 Aqueous solution (10ml) and brine (20ml), then washed with MgSO 4 dry. After removing the solvent under reduced pressure, the resulting solid was separated by column chromatography (eluent: CHCl 3 :CH 3 OH 9:1), to obtain 46mg of camptothecin-20-O-4-iodophenoxyacetate, yield 88.0%, mp 228-230°C.
[0415] Chemical structure analysis: 1 HNMR (CDCl 3 , 600MHz): δ8.41(s, 1H, Ar-H), 8.29(d, 1H, Ar-H), 7.98(d, 1H, Ar-H), 7.88(t, 1H, Ar-H), 7.7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com