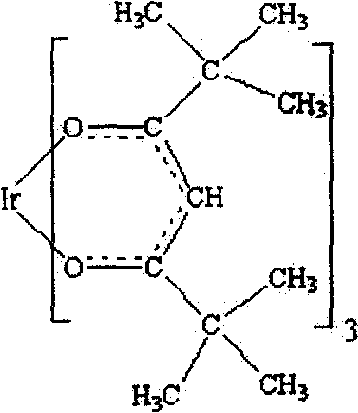
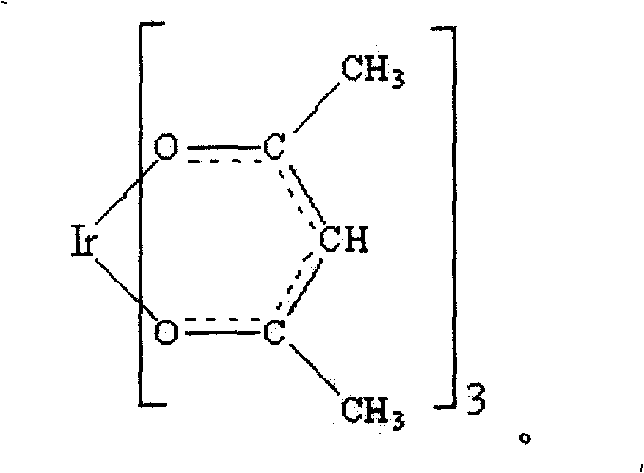
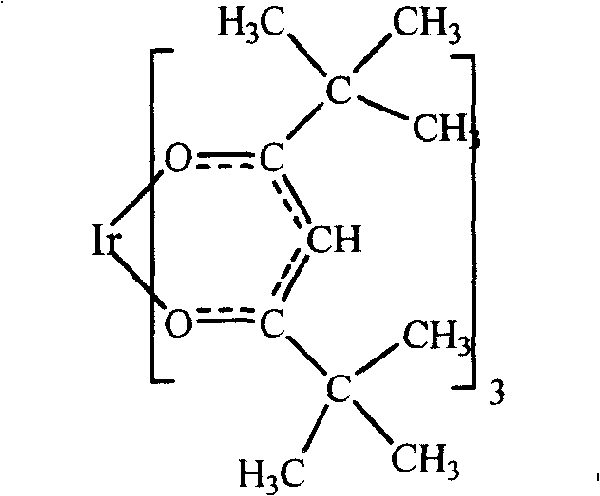
Iridium complex and its synthesis method
A technology for iridium complexes and synthesis methods, applied in chemical instruments and methods, compounds containing elements of Group 8/9/10/18 of the periodic table, organic chemistry, etc., can solve the problems of low volatility, low synthesis yield, etc. problem, to achieve the effect of increasing volatility, weakening interaction, and strengthening shading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1: take by weighing the chloroiridic acid solution of 1mmol, add 2mmol ascorbic acid, obtain the solution of trivalent iridium, reaction formula is as follows:
[0017]
[0018] Transfer to a 100ml three-necked flask; weigh 6mmol of the ligand 2,2,6,6-tetramethyl-3,5-heptanedione, dissolve it in absolute ethanol, then add 3ml of 2M ammonia solution, The reaction formula is as follows:
[0019]
[0020] The resulting solution was transferred to a separatory funnel, slowly added to a three-necked flask under stirring, stirred and heated at 85°C for 8 hours, and the reaction formula was as follows:
[0021]
[0022] After the reaction, place in a filter bottle to filter and wash until the solution is neutral. After vacuum drying, a yellow crude product was obtained; the yellow crude product to be obtained after recrystallization from benzene-hexane was dissolved in a benzene solvent and heated slightly to obtain a yellow translucent solution. Speed ...
Embodiment 2
[0023] Embodiment 2: take by weighing the chloroiridic acid solution of 1mmol, add 2mmol sodium nitrite, obtain the solution of trivalent iridium,
[0024] Ir 4+ +NO 2 - →Ir 3+ +NO 3 - (4)
[0025] Transfer to a 100ml three-necked flask; weigh 7mmol of the ligand 2,2,6,6-tetramethyl-3,5-heptanedione, dissolve it in absolute ethanol, and then add 2M sodium bicarbonate solution 3.5ml, the reaction formula is as follows:
[0026]
[0027] The resulting solution was transferred to a separatory funnel, slowly added to a three-necked flask under stirring, stirred and heated at 80°C for 10 hours, and the reaction formula was the same as in Example 1 (3); The solution is neutral. After vacuum drying, a yellow crude product was obtained; the 2,2,6,6-tetramethyl-3,5-heptanedione complex of iridium obtained after recrystallization from benzene-hexane was 0.1630 grams, and the 2 , 2,6,6-tetramethyl-3,5-heptanedione complex theoretical mass value is 0.7410 grams, the ratio of t...
Embodiment 3
[0028] Embodiment 3: take by weighing the chloroiridic acid solution of 1mmol, add 2mmol hydrazine hydrate, obtain the solution of trivalent iridium, reaction formula is as follows:
[0029] Ir 4+ +H 2 NNH 2 →Ir 3+ +N 2 ↑(6)
[0030] Transfer to a 100ml three-necked flask; weigh 8mmol of the ligand 2,2,6,6-tetramethyl-3,5-heptanedione, dissolve it in absolute ethanol, and then add 2M sodium bicarbonate solution 4ml, the resulting solution was transferred to a separatory funnel, and the reaction formula was the same as in Example 2 (5); under stirring, it was slowly added to a three-necked flask, stirred and heated at 95°C for 6 hours, and the reaction formula was shown in Example 1 (3). After the reaction, place it in a filter bottle to filter and wash until the solution is neutral. After vacuum drying, a yellow crude product was obtained; after recrystallization from benzene-hexane, 2,2,6,6-tetramethyl-3,5-heptanedione complex of iridium was obtained. The quality was 0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com