Process for preparing notoginseng diol saponin
A technology of diol saponins and alcohol saponins is applied in the field of preparation of extracting notoginseng glycol saponins from Panax notoginseng, and can solve the problems of the properties of notoginseng glycol saponins, the preparation method of pharmacological action, and the effects of human body, etc. The effect of low cost, stable quality and less loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0030] Example 1: Prepare according to the following steps:
[0031] 1. Take 100 grams of Panax notoginseng and crush it through the No. 1 sieve, add 60% ethanol to soak, percolate, and collect the percolate;
[0032] 2. Concentrate the percolate under reduced pressure at a temperature of 50°C to recover ethanol;
[0033] 3. Dilute the concentrated solution with distilled water to 500-600 ml, slowly add it to a macroporous adsorption resin separation column (the ratio of column diameter to column height: 30:160, with 100 ml of D101 resin inside), and then use 500 Milliliters of distilled water, 1000 milliliters of 40% ethanol and 1000 milliliters of 60% ethanol for elution, the flow rate is 0.5 times the bed volume / hour, and the 60% ethanol eluate is collected;
[0034] 4. The 60% ethanol eluate was concentrated under reduced pressure at a temperature of 50°C to a crude diol saponin solution with a relative density of 1.10;
[0035] 5. Mix the diol saponin crude product solu...
example 2
[0041] Example 2: Prepare according to the following steps:
[0042] 1. Take 50 grams of Panax notoginseng and crush it through the No. 1 sieve, add 60% ethanol to soak, percolate, and collect the percolate;
[0043] 2. Concentrate the percolate under reduced pressure at a temperature of 50°C to recover ethanol;
[0044] 3. Dilute the concentrated solution to 250-300 ml with distilled water, centrifuge and filter, and slowly add the aqueous solution to the macroporous adsorption resin separation column (the ratio of column diameter to column height: 30:160, with 40 ml of D101 resin inside) In, eluted with 200 milliliters of distilled water, 300 milliliters of 40% ethanol and 400 milliliters of 60% ethanol successively, the flow rate is 0.6 times bed volume / hour, collects 60% ethanol eluate;
[0045] 4. The 60% ethanol eluate was concentrated under reduced pressure at a temperature of 50°C to a crude diol saponin solution with a relative density of 1.10;
[0046] 5. Mix the d...
example 3
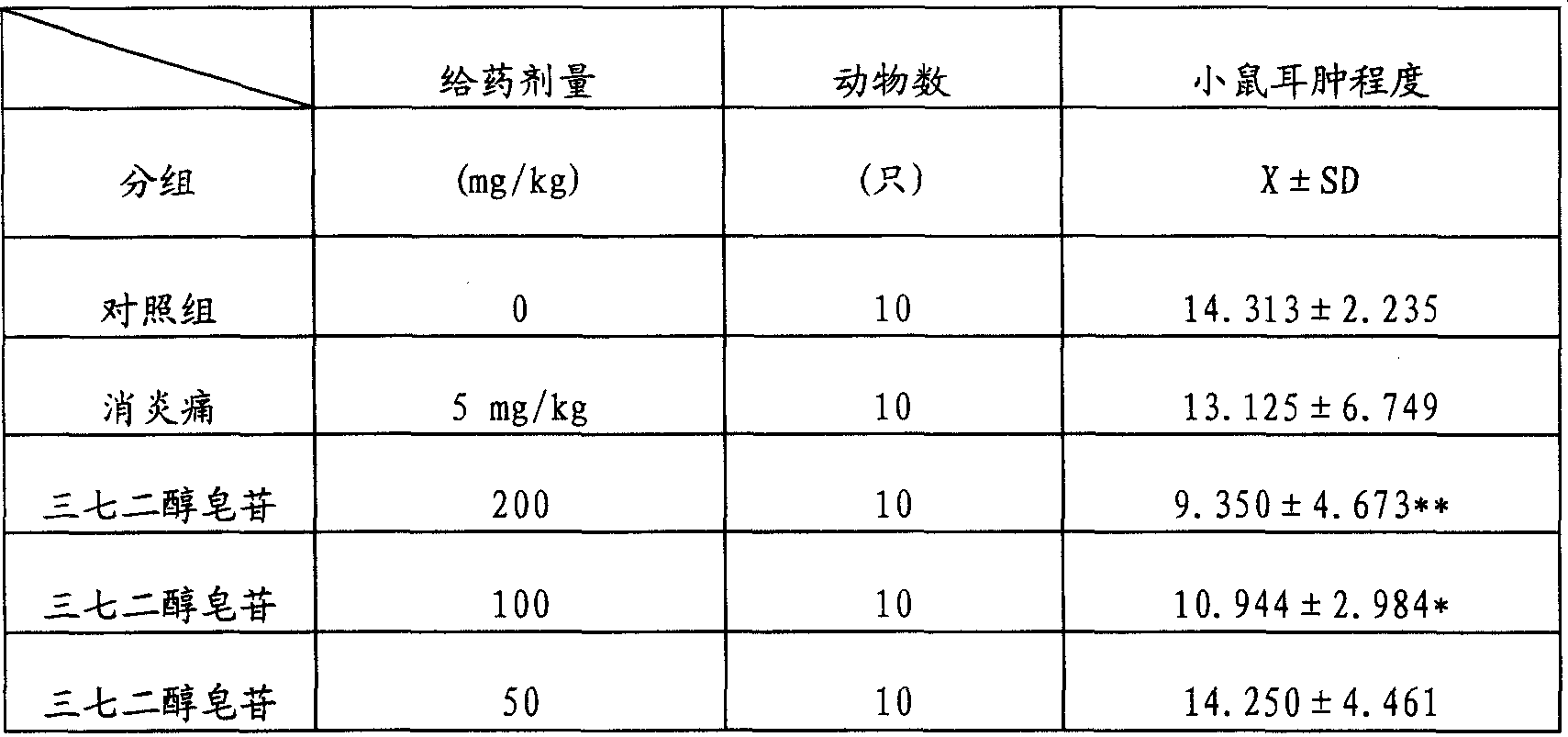
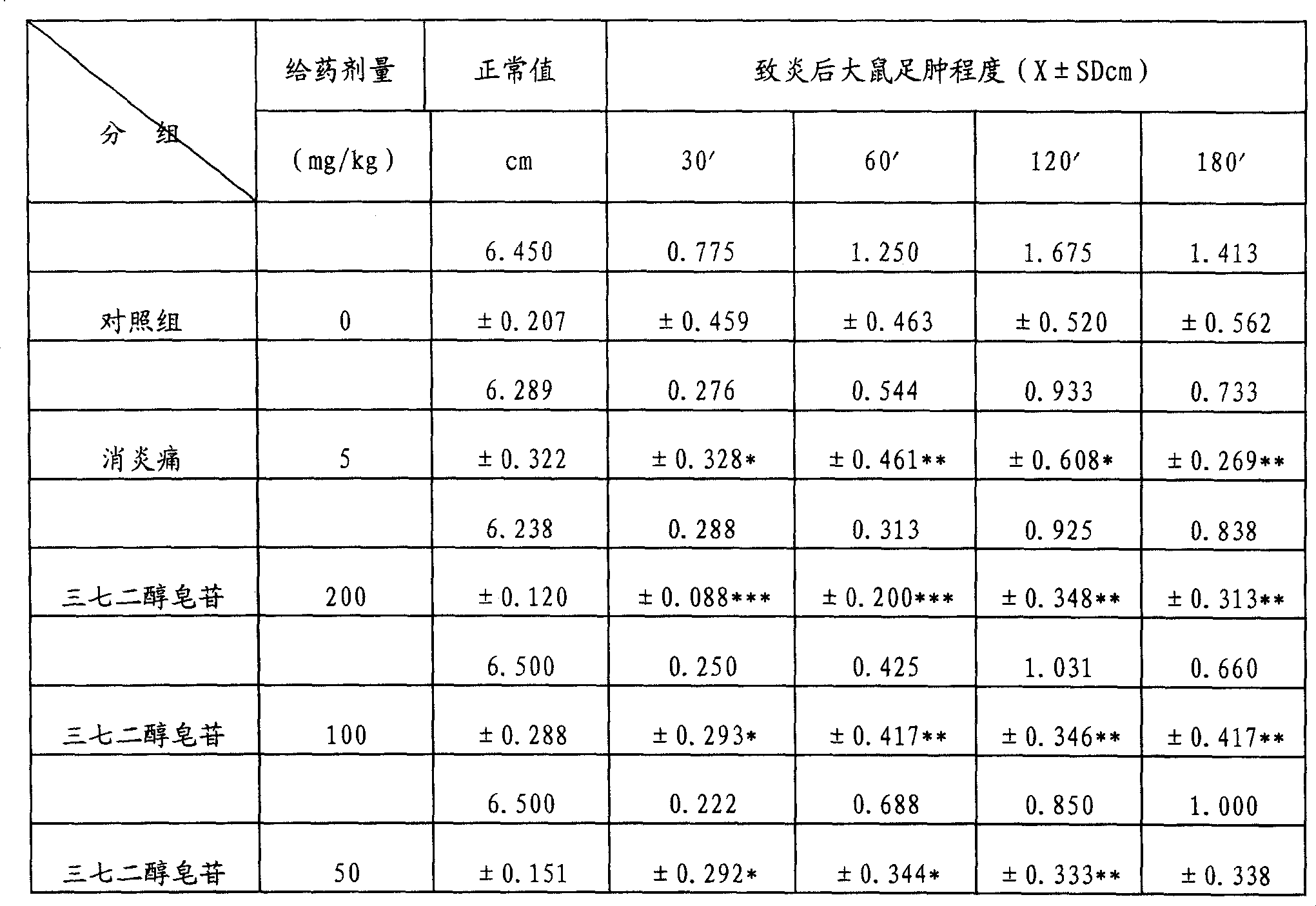
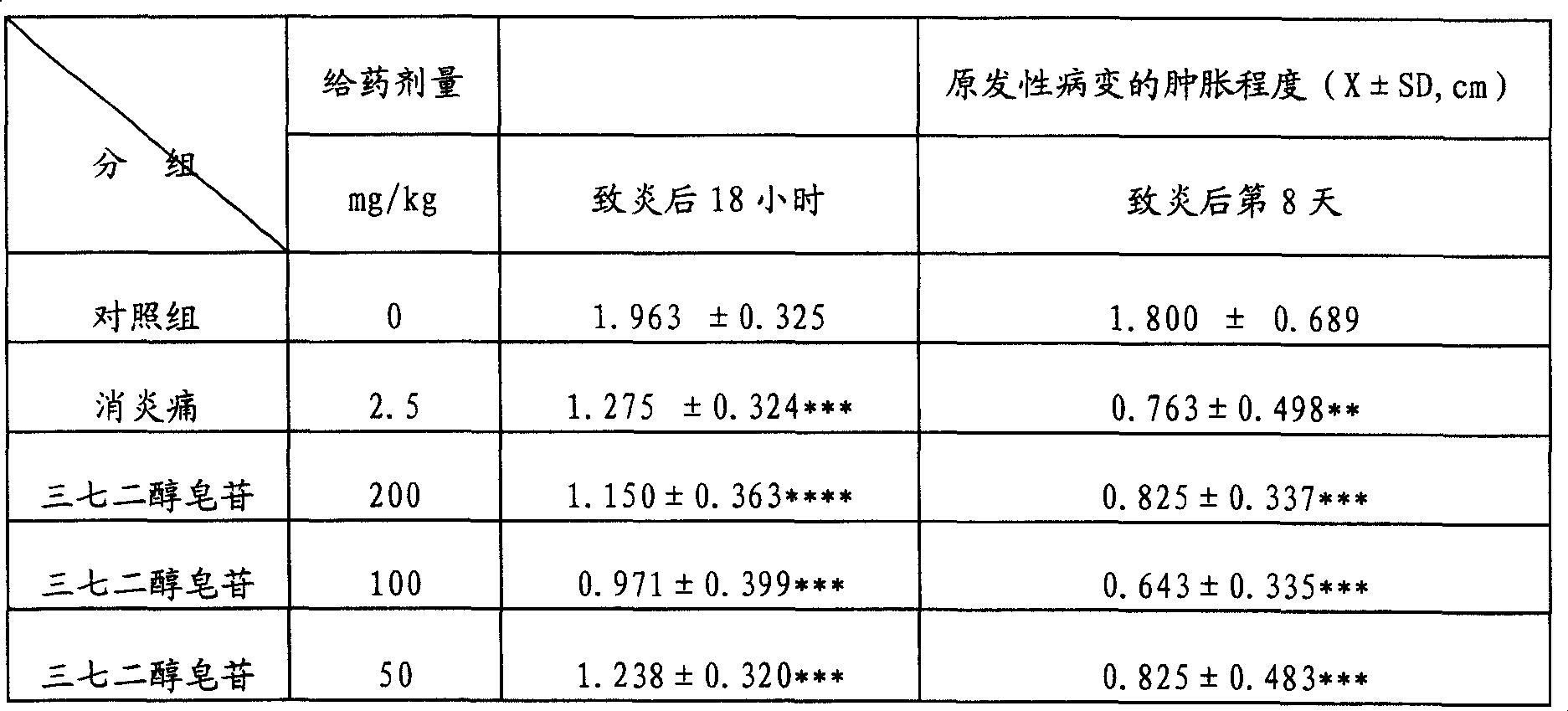
[0052] Example 3: Anti-inflammatory and Analgesic Effects of Notoginsengdiol Saponins
[0053] The test drug notoginsengdiol saponin was provided by the Phytochemical Department of Tianjin Pharmaceutical Research Institute as a light yellow-brown powder batch number 940201, with a content of R b1 =59.15 Rd=15.10%, before the test, it was prepared into a solution of the required concentration with distilled water, and it was given to the experimental animals for intragastric administration.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com