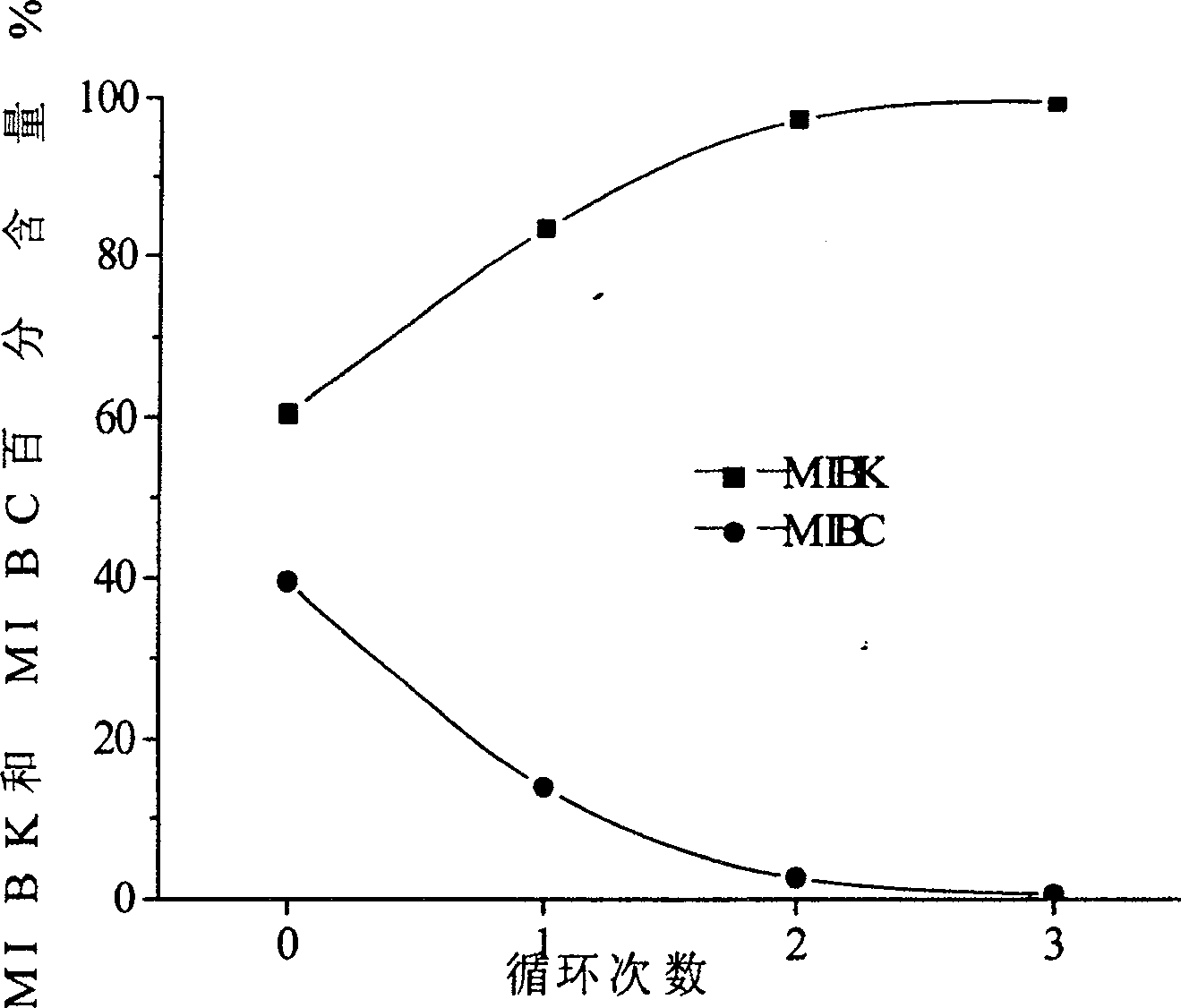
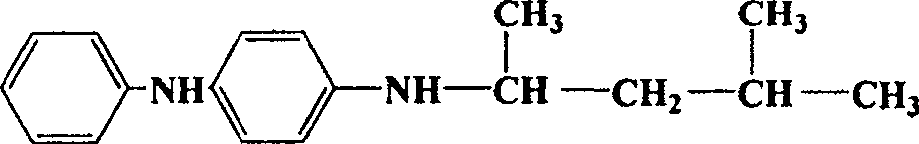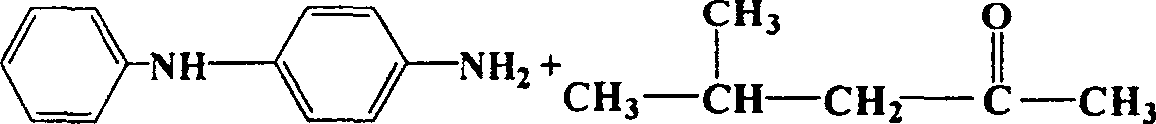Copper catalyst for vapor catalytic dehydrogenation of methyl isobutyl alcohol and its preparation process and application method
A technology of methyl isobutyl alcohol and copper-based catalysts, which is applied in the direction of catalyst activation/preparation, metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, etc., to achieve good dehydrogenation stability and preparation The method is mature and the effect of long life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: Preparation of copper-based catalyst 1 by co-precipitation method
[0032] 5 parts Cu(NO 3 ) 2 ·3H 2 O, 10 parts of Zn(NO 3 ) 2 ·6H 2 O, 5 parts of Al(NO 3 ) 3 9H 2 O, 90 parts of deionized water, stir to make it evenly mixed, and then heat to 80°C. 20 parts Na 2 CO 3 , 80 parts deionized water, stirred to get 20% Na 2 CO 3aqueous solution. Constantly stir the above nitrate aqueous solution, add Na dropwise within 40min 2 CO 3 Solution, make it fully react, and keep stirring at the same temperature for 1-2h, check the pH value of the solution with pH test paper, add an appropriate amount of Na 2 CO 3 Solution Adjust the pH of the solution to 7.0. The obtained precipitate was aged and filtered, washed several times with deionized water, dried at 110°C, calcined at 300-450°C for 4 hours, and formed into a black cylindrical granular catalyst with a diameter of Φ5×5mm.
Embodiment 2
[0033] Embodiment 2: coprecipitation method prepares copper-based catalyst 2
[0034] 10 parts of Cu(NO 3 ) 2 ·3H 2 O, 5 parts of Zn(NO 3 ) 2 ·6H 2 O, 1 part of Al(NO 3 ) 3 9H 2 O, 83 parts of deionized water, stir to mix evenly, and then heat to 80°C. 10 parts Na 2 CO 3 , 90 parts deionized water, stirred to get 10% Na 2 CO 3 aqueous solution. Constantly stir the above nitrate aqueous solution, add Na dropwise within 40min 2 CO 3 Solution, make it fully react, and keep stirring at the same temperature for 1-2h, check the pH value of the solution with pH test paper, add an appropriate amount of Na 2 CO 3 Solution Adjust the pH of the solution to 7.0. The obtained precipitate was aged and filtered, washed several times with deionized water, dried at 110°C, calcined at 300-450°C for 4 hours, and formed into a black cylindrical granular catalyst with a diameter of Φ5×5mm.
Embodiment 3
[0035] Embodiment 3: coprecipitation method prepares copper-based catalyst 3
[0036] 7 parts Cu(NO 3 ) 2 ·3H 2 O, 3 parts of Zn(NO 3 ) 2 ·6H 2 O, 1 part of Al(NO 3 ) 3 9H 2 O, 89 parts of deionized water, stirred to make it evenly mixed, and then heated to 80°C. 5 Na 2 CO 3 , 95 parts deionized water, stirred to get 5% Na 2 CO 3 aqueous solution. Constantly stir the above nitrate aqueous solution, add Na dropwise within 40min 2 CO 3 Solution, make it fully react, and keep stirring at the same temperature for 1-2h, check the pH value of the solution with pH test paper, add an appropriate amount of Na 2 CO 3 Solution Adjust the pH of the solution to 7.0. The obtained precipitate was aged and filtered, washed several times with deionized water, dried at 120°C, calcined at 300-450°C for 4 hours, and formed into a black cylindrical granular catalyst with a diameter of Φ5×5mm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com