Tocopherol derivatives with a long hydroxylated chain, which can be used as neurotrophics
A hydroxyl and compound technology, applied in the application field of compounds of general formula and pharmaceutical compositions, can solve the problems of efficiency and application limitations, the inability of protein growth factors to penetrate various biological barriers, and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
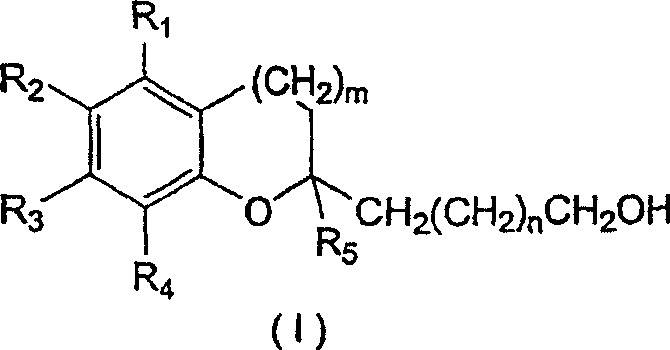
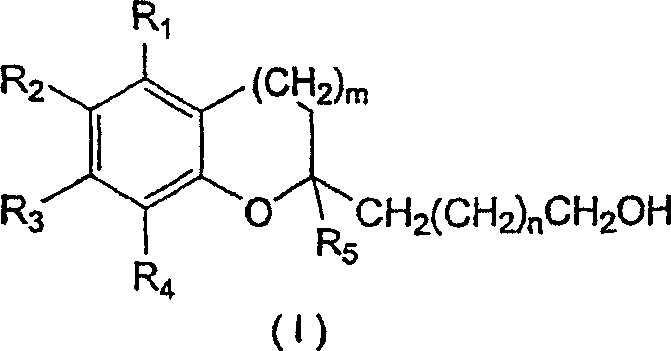
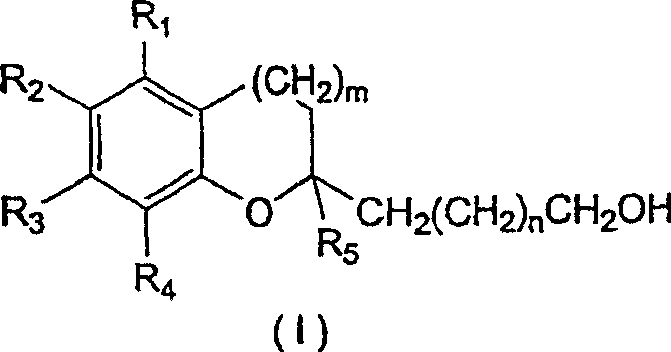
[0062] A. Preparation of Compounds of Structural Formula (I)
[0063] 1. Preparation of N-methoxy-N-methyl-16-hydroxyhexadecanoamide
[0064] 1.78 g of dimethylhydroxylamine (17.687 mmol; 3 eq.; molecular weight 97.55) were dissolved in 10 ml of dichloromethane which had been distilled under argon and cooled to -78°C. At -78°C, 8.8 ml of 2M trimethylaluminum in hexane (17.687 mmol; 3 eq.; molecular weight 72.09) was added dropwise, and the reaction mixture was stirred at room temperature for half an hour. The solution was then cooled to 0° C. and 1.55 g of oxacycloheptadecan-2-one (5.895 mmol, 1 equiv; molecular weight 254.42) dissolved in 5 ml of distilled dichloromethane was added dropwise. The reaction mixture was stirred at room temperature. TLC analysis indicated complete reaction after 1.5 hours. The solution was added dropwise to 60 mL of a 2:1 diethyl ether / methanol mixture cooled to -78 °C and filtered through celite. 100 ml of saturated aqueous sodium bicarbonate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com