Disposal systems of transdermal delivery devices to prevent misuse of the active agents contained therein
A technology of disposal system and drug delivery device, which is applied in the fields of nervous system diseases, organic active ingredients, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
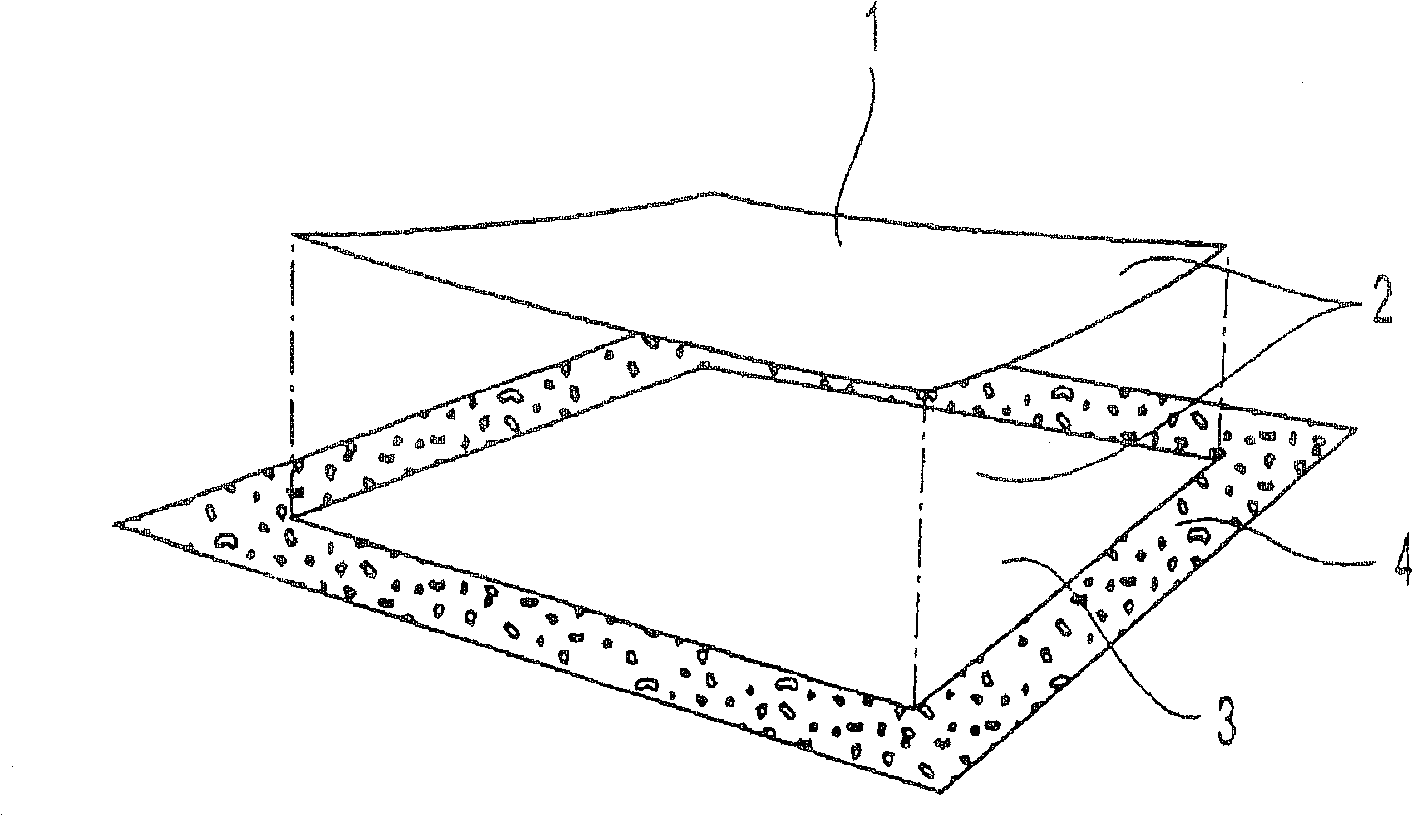
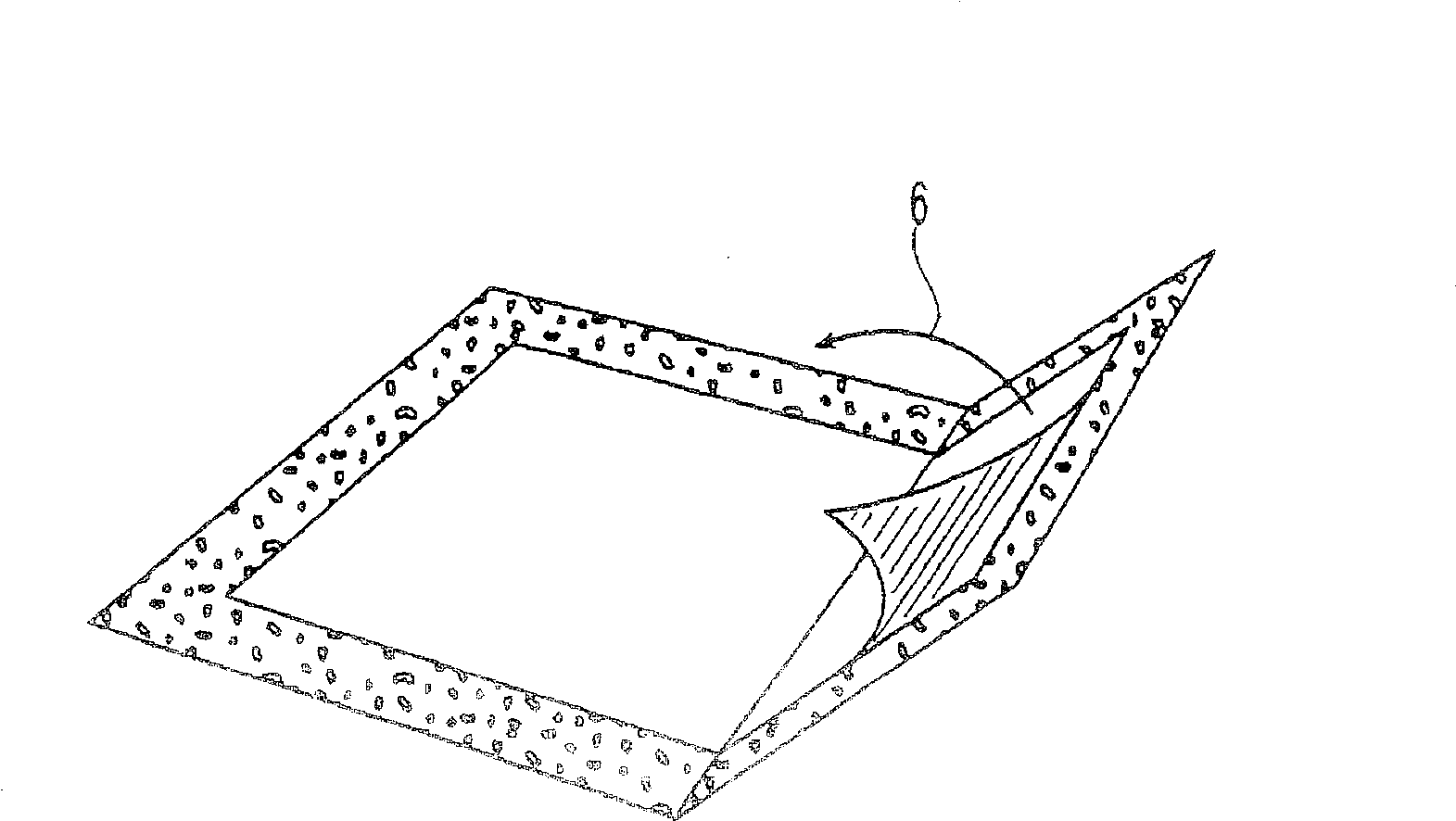
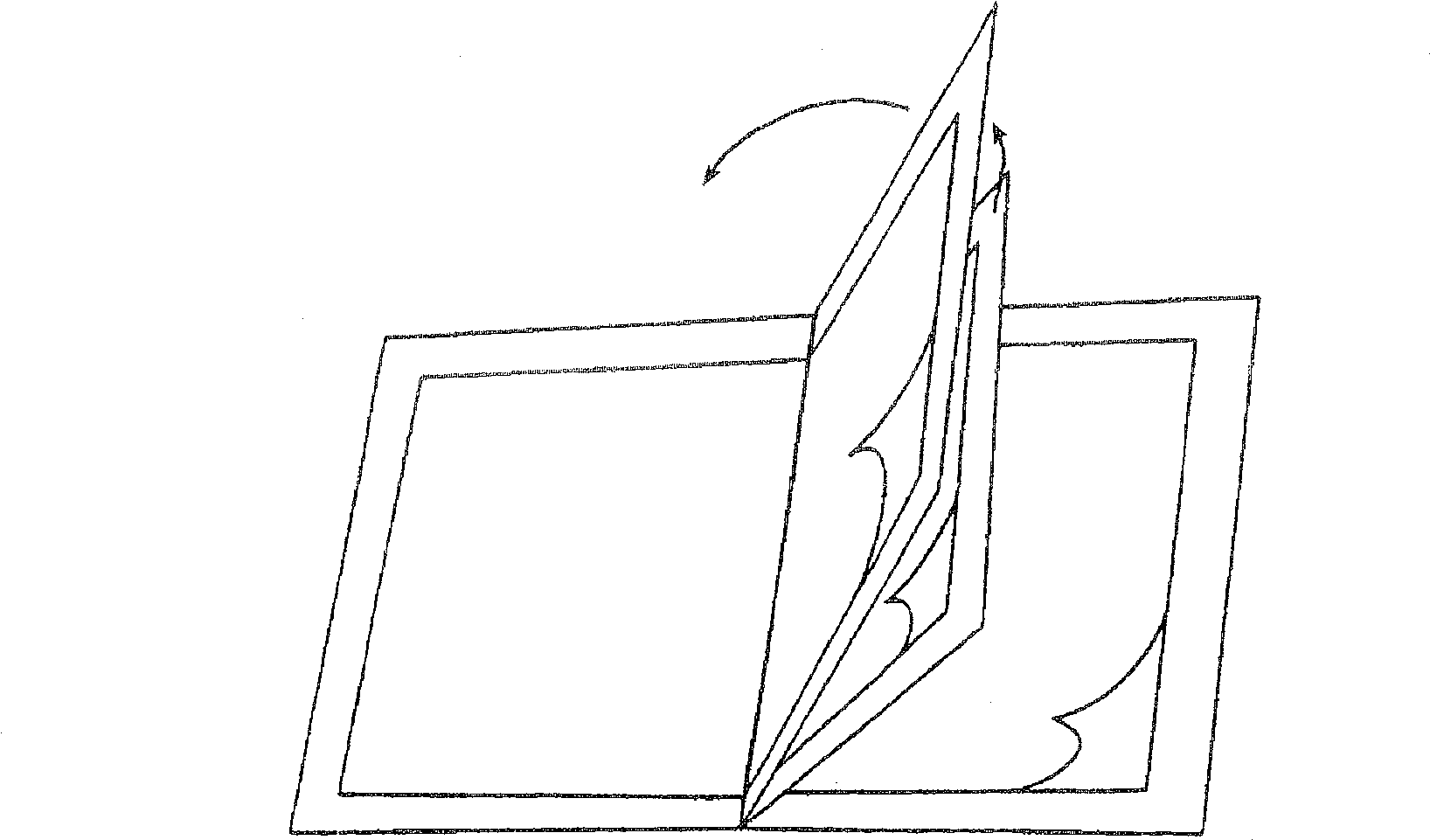
[0152] In Example 1, the transdermal applicator was loaded with buprenorphine (BUP) as shown in FIG. 2B . The specific BUP TDD used in Example 1 was similar in composition to those marketed in Europe under the trade name TRANSTEC by Gruenenthal of Switzerland, but it only contained a total BUP dose of 20 mg (and therefore had a different surface area than the commercially available TDD). The BUP TDD was then fixed and isolated using the TDD disposal system as shown in Figure 1B. The amount of BUP extractable (and thus potentially available for abuse) in some cases from the TDD contained in the TDD disposal system was then determined by extraction using the following solvents: distilled water, tap water, 0.026 M sodium bicarbonate in water, 5 % vinegar, acetone, methanol, ethanol, ethyl acetate or ether. Extraction was performed under the following extraction conditions: after 5 min, 60 min and 120 min in the extraction solvent at room temperature of about 25°C. For each extr...
Embodiment 2
[0175] For the pilot Example 2, the transdermal applicator was loaded with BUP as shown in Figure 2B, as in Example 1. The BUP TDD was then fixed and isolated using the TDD disposal system as shown in Figure 1C. The amount of BUP extractable (and thus potentially available for abuse) in some cases from the TDD contained in the TDD disposal system was then determined by extraction using the following solvents: distilled water, tap water, 0.026M aqueous sodium bicarbonate, 5% vinegar, acetone, methanol, ethanol, ethyl acetate and / or diethyl ether. Extraction was performed under the following extraction conditions: room temperature at about 25°C, and after 5 min, 60 min and 120 min in the extraction solvent at reflux (ie, at about the boiling temperature of the extraction solvent). For each extraction solvent, the TDD exists in the following isolated state: (1) the first substrate (i.e., pasted to the TDD) is not pasted on the second substrate (adhesive side of the sticking devi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com