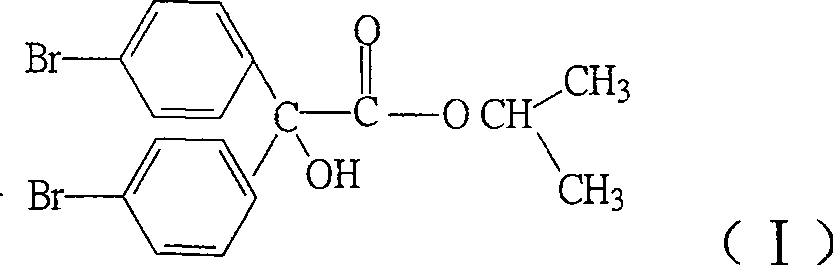
Method for synthesizing akarol fenisobromolate
A synthesis method and technology of bromodifen, applied in the field of the synthesis of acaricides, bromodifen, can solve the problems of high cost, low yield, long synthesis process route, etc., and achieve low cost, high yield, favorable The effect of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
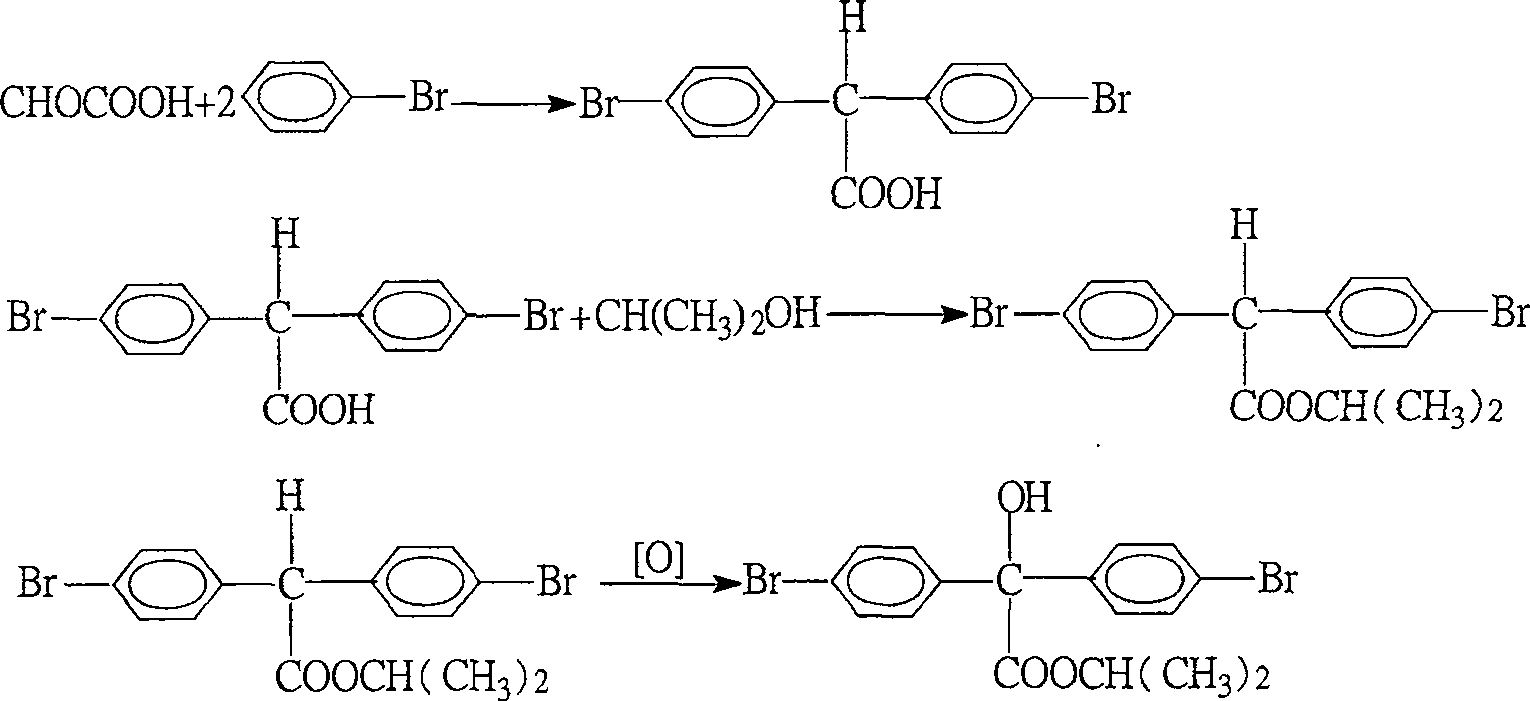
[0027] Embodiment 1: the preparation of 4,4'-dibromodiphenylacetic acid
[0028] Put 72g of glyoxylic acid into a 500mL four-neck flask, distill under reduced pressure to remove 40g of water (glyoxylic acid contains 60% water, the same below), cool to 0-5°C, and add 45g of chlorosulfur dropwise within 30 minutes Acid (tail gas water absorption), continue to stir for 15 minutes, add 140g of bromobenzene dropwise within 15 minutes, then add 45g of chlorosulfonic acid dropwise again within 2.0 hours. Raise the temperature to 25°C and keep it warm for 20 hours. After the reaction was finished, 150 g of water was added dropwise into the reaction mixture, and the excess bromobenzene was distilled off by heating up. After cooling, the water was sucked off, and the residue was recrystallized with toluene to obtain 90 g of 4,4'-dibromodiphenylacetic acid with a content of more than 95% and a yield of 59.4%.
Embodiment 2
[0029] Embodiment 2: Preparation of 4,4'-dibromodiphenylacetic acid
[0030] Put 72g of glyoxylic acid into a 500ml four-neck flask, remove 40g of water by distillation under reduced pressure, cool to 0-5°C, add 40ml of acetic acid dropwise within 30 minutes, continue stirring for 15 minutes, and add 140g of bromobenzene within 15 minutes. Then 95 g of chlorosulfonic acid were added dropwise over 2.0 hours. Raise the temperature to 25°C and keep it warm for 20 hours. After the reaction was finished, 150 g of water was added dropwise to the reaction mixture, and the excess bromobenzene was evaporated by heating up. After cooling, the water was sucked off, and the residue was recrystallized with toluene to obtain 92g of 4,4'-dibromodiphenylacetic acid with a content of more than 95% and a yield of 60.7%.
Embodiment 3
[0031] Embodiment 3: Preparation of 4,4'-dibromodiphenylacetic acid
[0032] Put 72g of glyoxylic acid into a 500ml four-neck flask, remove 40g of water by distillation under reduced pressure, cool to 0-5°C, add 50g of 98% concentrated sulfuric acid dropwise within 30 minutes, continue stirring for 15 minutes, and add 140g bromobenzene. Then, 85 g of chlorosulfonic acid were added dropwise over 2.0 hours. Raise the temperature to 25°C and keep it warm for 20 hours. After the reaction was finished, 160 g of water was added dropwise to the reaction mixture, and the excess bromobenzene was evaporated by heating up. After cooling, the water was sucked off, and the residue was recrystallized with toluene to obtain 98g of 4,4'-dibromodiphenylacetic acid with a content of more than 95% and a yield of 64.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com