Modified vaccinia ankara virus variant
A vaccinia virus and vaccine technology, applied in the direction of viruses/phages, viruses, viral peptides, etc., can solve the problems of absolute safety, loss of replication competitiveness, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
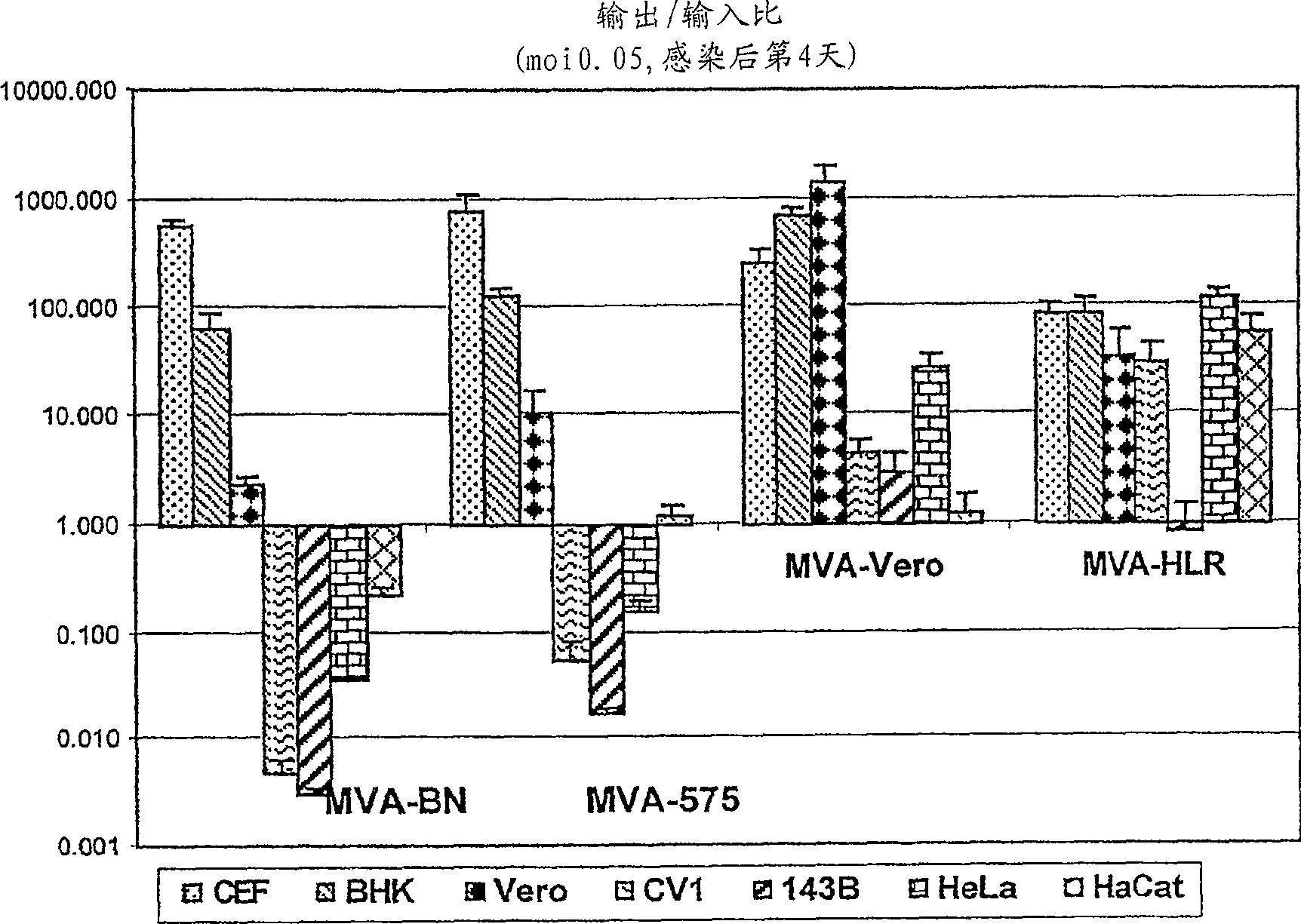
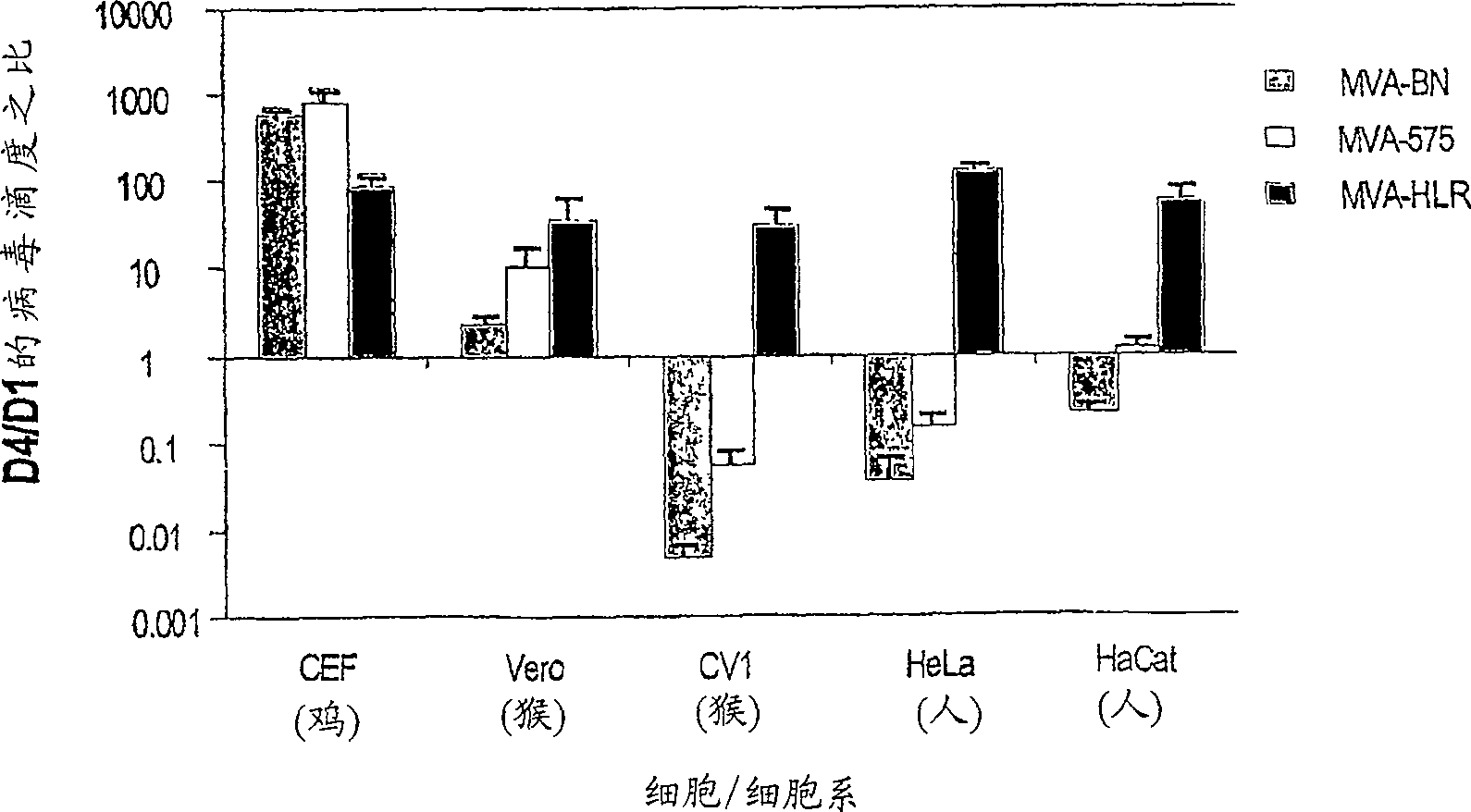
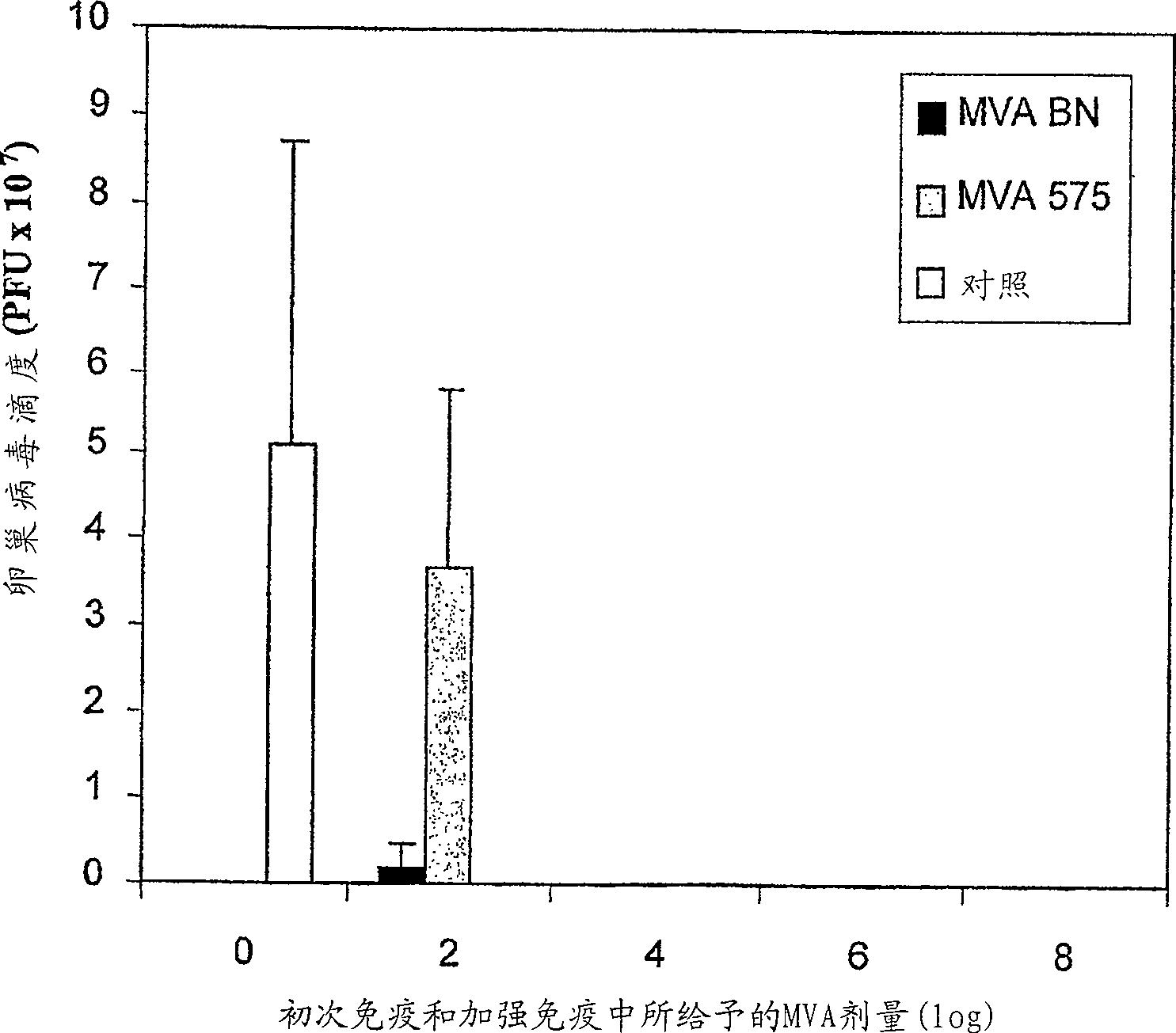
[0014] As shown in detail in Example 1 and Table 1, the viruses of the present invention are unable to reproduce reproductively in the cell lines 143B, HeLa or HaCat. The specific virus strains used in the examples of the present invention have been deposited in the European Collection of Cell Cultures with the accession number V00083008. This strain is referred to throughout the specification as "MVA-BN".
[0015] Known MVA strains showed residual replication in at least one of the human cell lines tested (Figure 1, Example 1). All known vaccinia virus strains show at least partial replication in the cell line HaCat, whereas the MVA virus strains of the present invention, especially MVA-BN, do not reproduce reproductively in HaCat cells. More specifically, MVA-BN showed an expansion ratio of 0.05-0.2 in the human embryonic kidney cell line 293 (ECACC 85120602). In the human bone osteosarcoma cell line 143B (ECACC 91112502), the expansion ratio was 0.0-0.6. In the human cer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com