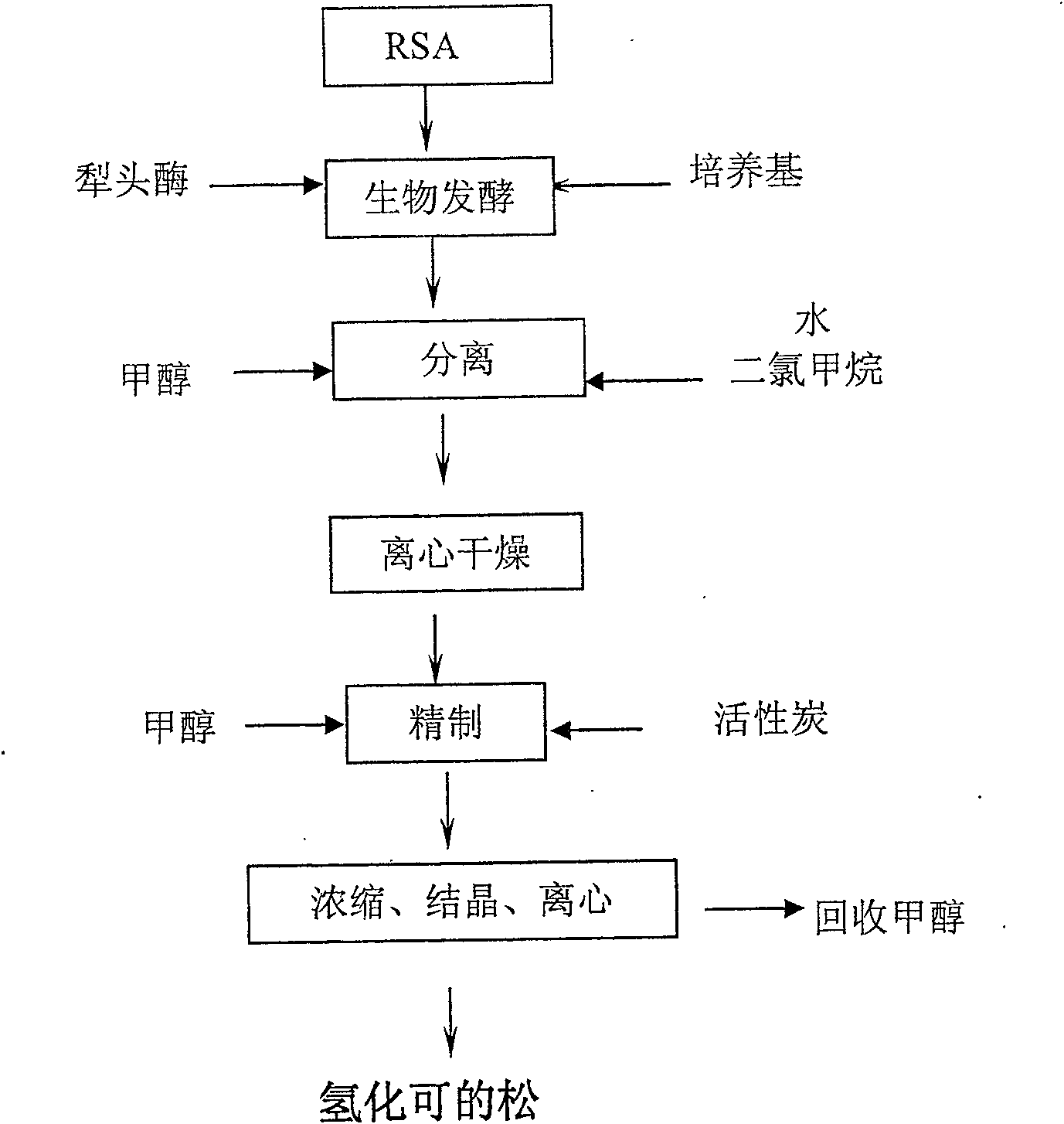
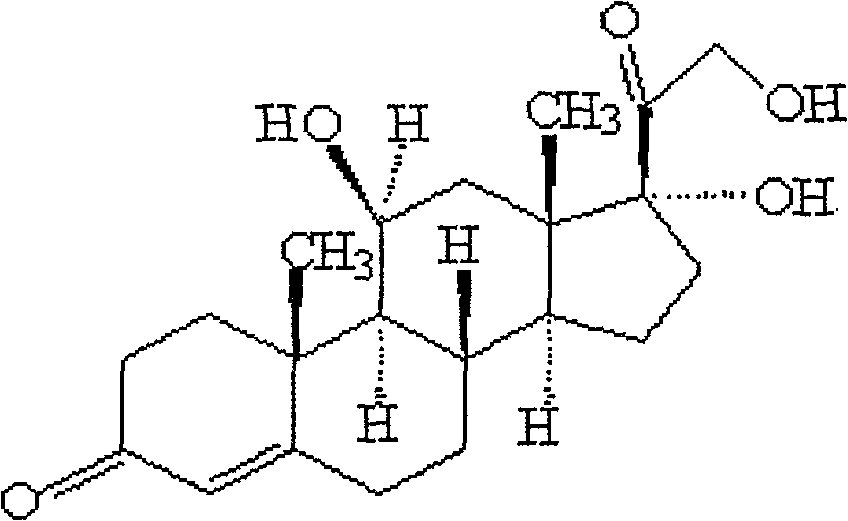
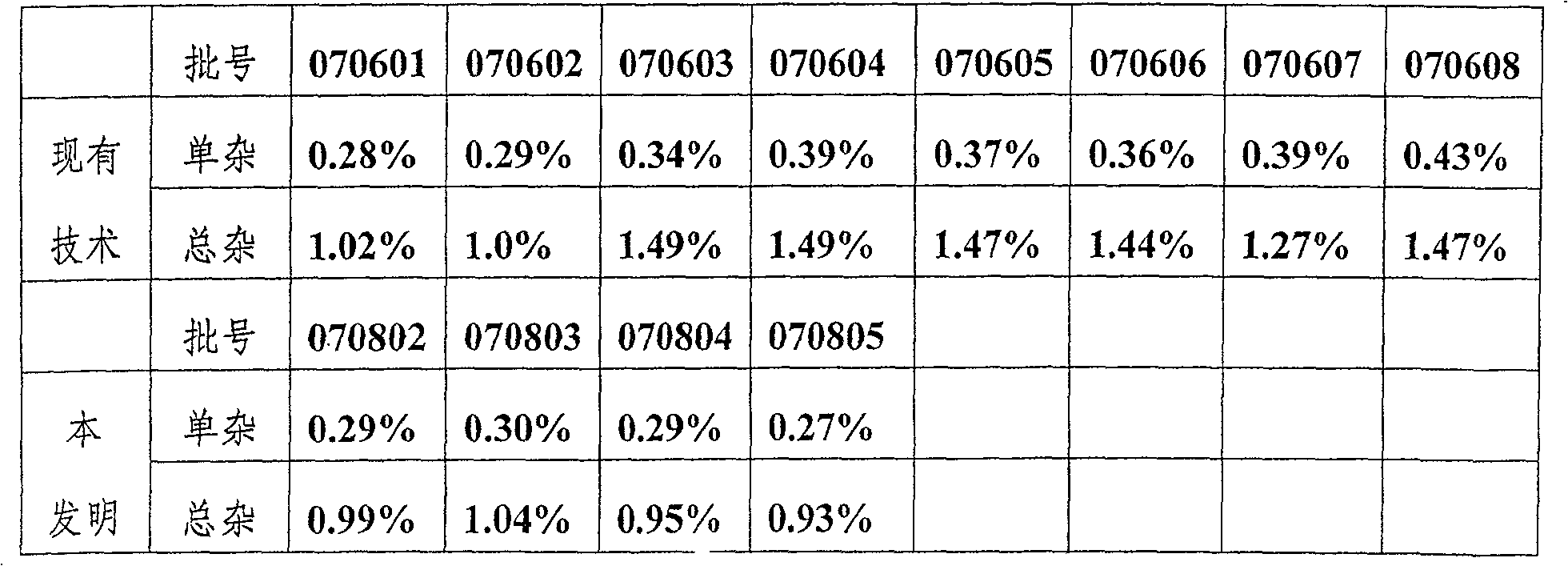
Preparation technique for high-purity hydrocortisone
A technology of hydrocortisone and preparation process, which is applied in the directions of organic chemistry, steroids, etc., can solve the problems of high toxicity to operators, inconvenient procurement and transportation, and inability of medicines to pass through, so as to achieve low comprehensive cost and low toxicity. , safe to use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Put 30g of crude hydrocortisone, 358g of dichloromethane, and 95g of methanol into the reaction tank for one separation, stir, heat at 32°C and reflux for 20min, cool down to 0°C and keep for 20min, add 105g of water to dilute, continue to cool down for 1 hour, let stand for 1 hour, and centrifuge Dry put into secondary separation, add 135g of dichloromethane, 36g of methanol, stir, heat at 32°C and reflux for 20min, cool down to 0°C and keep for 20min, add 54g of water to dilute, continue to cool down for 1 hour, let stand for 1 hour, and centrifuge and dry to obtain hydrocortisone isolate 12.7g. The quality is acceptable.
Embodiment 2
[0026] Put 30g of crude hydrocortisone, 199g of dichloromethane, and 50.4g of methanol into the reaction tank for one separation, stir, heat at 32°C and reflux for 20min, cool down to 0°C and keep for 20min, add 105g of water to dilute, continue to cool down for 1 hour, let stand for 1 hour, and centrifuge Spin dry and put into secondary separation, add 120.6g of dichloromethane, 28.8g of methanol, stir, heat at 32°C and reflux for 20min, cool down to 0°C and keep for 20min, add 55g of water to dilute, continue to cool down for 1 hour, let it stand for 1 hour, and centrifuge and dry to obtain hydrogenated cocoa The loose isolate was 17.5g, and the quality was qualified.
Embodiment 3
[0028] Put 30g of crude hydrocortisone, 240g of dichloromethane, and 60g of methanol into the reaction tank for one separation, stir, heat at 32°C and reflux for 20min, cool down to 0°C and keep for 20min, add 105g of water to dilute, continue to cool down for 1 hour, let stand for 1 hour, and centrifuge Dry put into secondary separation, add 126g of dichloromethane, 32.4g of methanol, stir, heat at 32°C and reflux for 20min, cool to 0°C and keep for 20min, add 60g of water to dilute, continue to cool down for 1 hour, stand still for 1 hour, and centrifuge and dry to obtain hydrocortisone separation The product is 16.9g, and the quality is qualified.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com