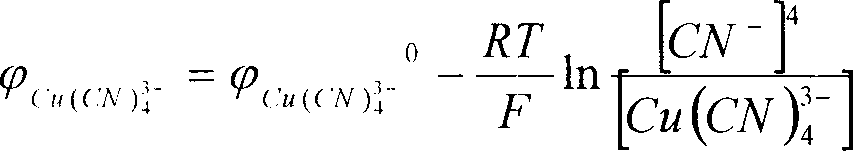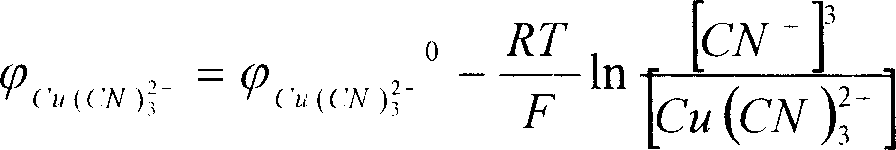Method for recovering copper from cyanide-containing waste water and related waste water treatment method
A disposal method and technology for copper recovery, applied in water/sewage treatment, water/sludge/sewage treatment, chemical instruments and methods, etc., can solve the problems of long process and low current efficiency, and achieve closed-circuit zero discharge, avoid Effects of Oxidative Loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The feed liquid is a poor cyanide solution, and its composition is as follows: CNt 14.57g / L, Cu 8.1g / L, Zn 2.5g / L, CN4.0g / L, SCN- 6.56g / L, Fe trace, pH=11.9 .
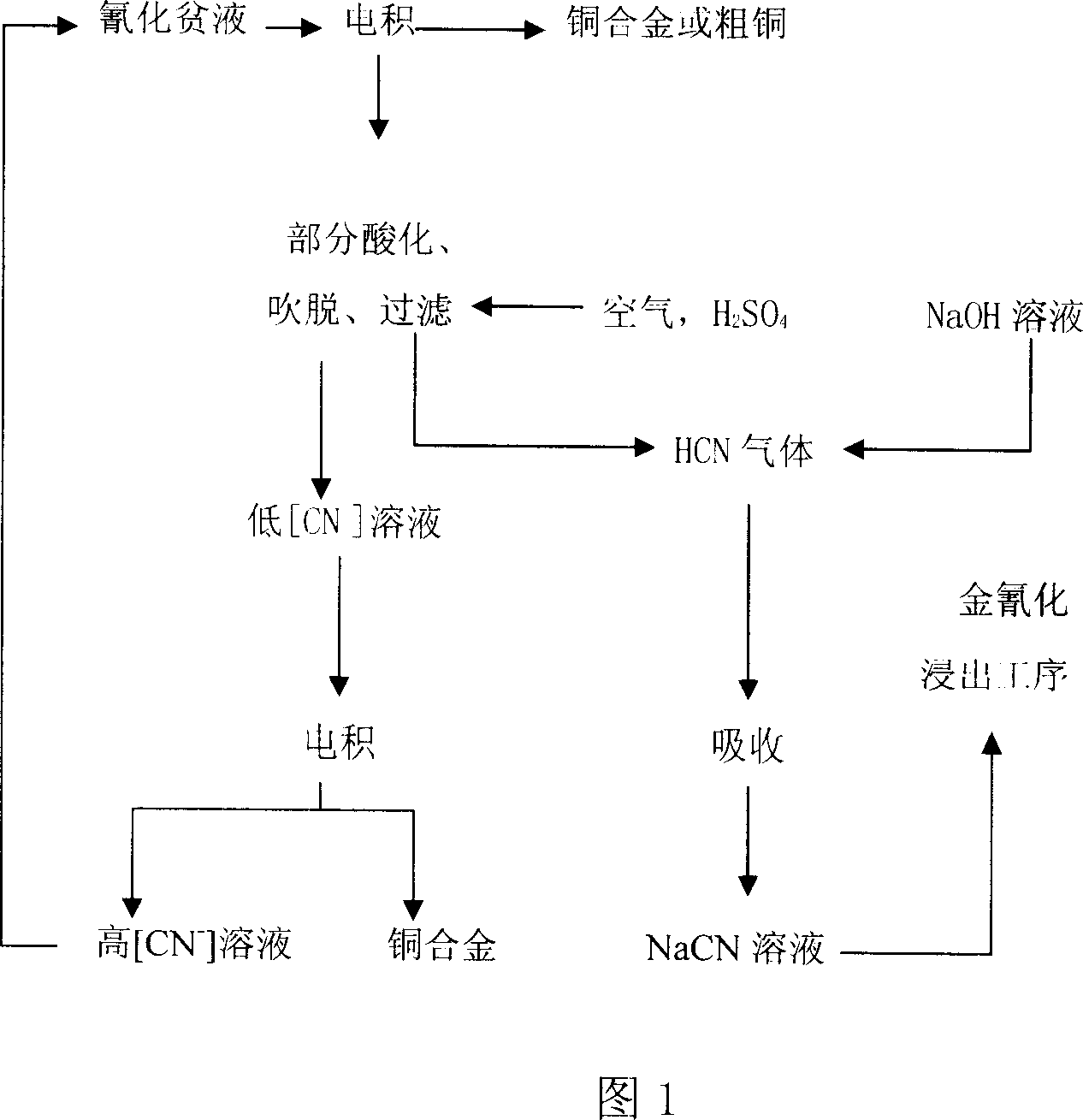
[0039] When the solution temperature is 38°C, pH=9, the current density is 9.8~12mA / cm 2 Under the electrowinning conditions, brass containing 73% copper can be obtained on the cathode in the first 2 hours, and then blister copper containing 97% copper can be obtained by continuing electrowinning. Table 1 lists certain cathode blister copper compositions.
[0040] Table 1 cathode blister copper composition (%)
[0041] Cu,
Zn
Ca
Pb
Si
Fe
other
97.0
2.5
0.21
0.17
0.038
0.023
0.059
[0042]When the current efficiency drops below 40% (about 4 to 5 hours), pump the electrolytic solution into the acidification tank, and add sulfuric acid with a concentration of 50% (v / v) into the electrolytic solution at a volume rat...
Embodiment 2
[0046] The feed liquid is a high-concentration copper-cyanide solution, and its composition is as follows: CNt 64g / L, Cu 30.0g / L, Zn 4.1g / L, CN 20g / L, Fe 0.1g / L, pH=12.
[0047] The process flow of electrowinning-partial acidification is shown in Figure 1.
[0048] At a solution temperature of 45°C, pH=10, and a current density of 20-40mA / cm 2 Brass can be obtained by electrodeposition under certain conditions; when the solution temperature is higher than 44°C, the pH is 4.5-8, and the current density is 10-25mA / cm 2 Blister copper containing copper ≥ 98.0% can be obtained by electrowinning. Table 2 lists certain cathode brass and blister copper compositions.
[0049] Table 2 Composition of cathode brass and blister copper (%)
[0050] Cu,
Zn
Ca
Pb
Fe
other
70.5
29.1
0.19
0.1
0.01
0.10
98.2
1.4
0.20
0.1
0.02
0.08
[0051] When the current ef...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com