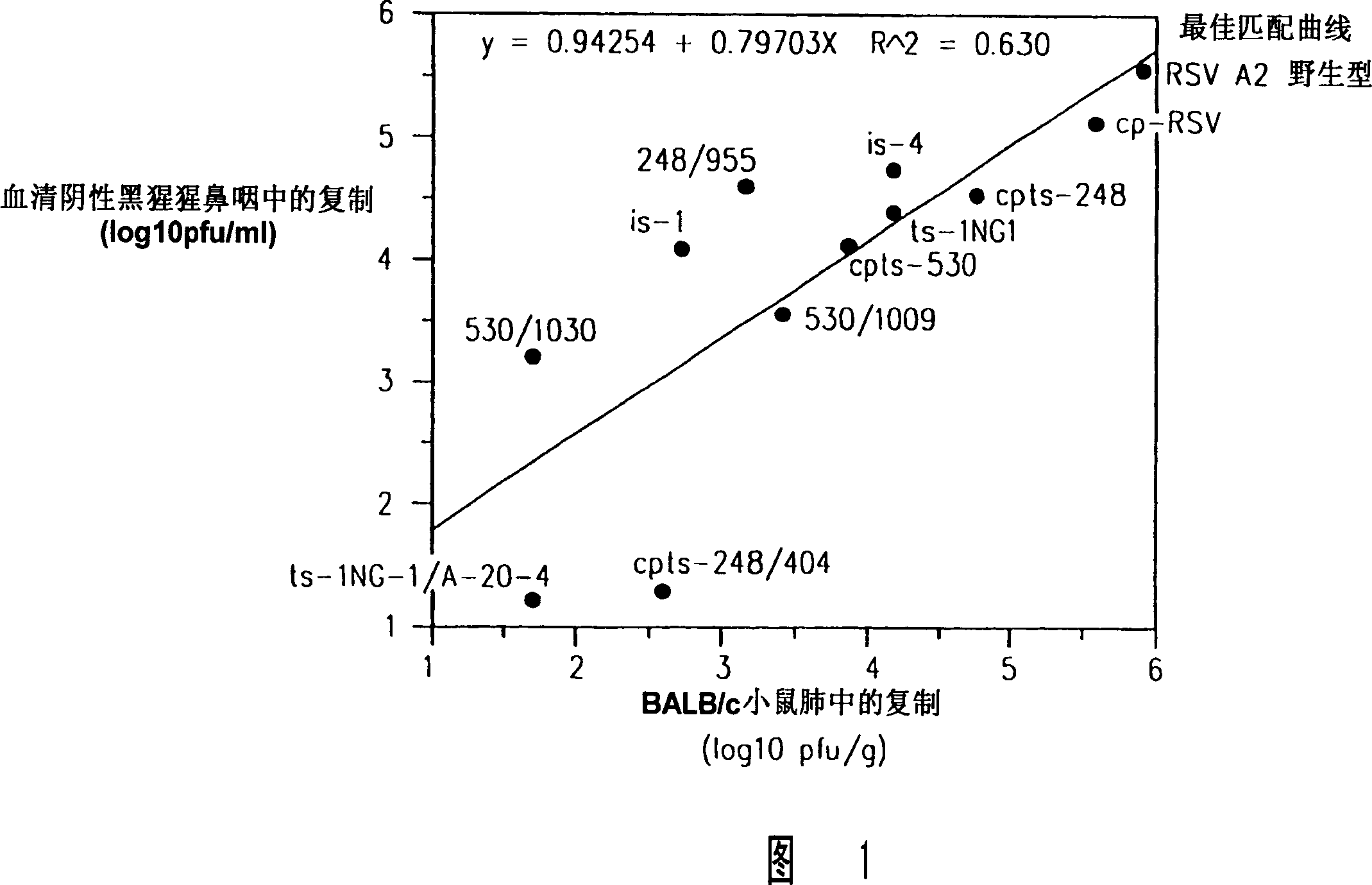
Production of attenuated chimeric respiratory syncytial virus vaccines from cloned nucleotide sequences
一种合胞病毒、呼吸道的技术,应用在从克隆的核苷酸序列制备减毒呼吸道合胞病毒疫苗领域,能够解决感染性RSV拯救复杂等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
[0137] Isolation and Identification of Mutagenized Derivative Strains of Cold Passage RSV
[0138] This example describes the chemical mutagenesis of incompletely attenuated, host range-restricted cpRSV to produce derivative ts and sp mutants with higher attenuation, which are suitable for the preparation of RSV vaccines.
[0139] Preparation of parental stocks of cold passaged RSV (cpRSV). Make Flow Laboratories Lot 3131 virus (the cpRSV parental virus of incomplete attenuation in the human body) go down twice in 25 ℃, MRC-5 cell, 25 ℃, terminally dilute (terminally dilute) twice in MRC-5 cell, then in MRC-5 was passaged three times to generate a cpRSV suspension for mutagenesis.
[0140] Make the parent stock in MRC-5 cells at 32°C with a concentration of 4×10 -4 Growth in M 5-fluorouracil medium induces cpRSV mutation. Basic studies have proven this concentration to be optimal, based on a 100-fold reduction in viral titer compared to media without ...
Embodiment II
[0264] Attenuation of cpRSV mutant strains by cold adaptation
[0265] This example describes the introduction of growth-limiting mutations into incompletely attenuated, host-range-restricted cpRSV strains by further passage of the strains at progressively lower temperatures to produce more satisfactorily attenuated human vaccines. Derivatives.
[0266] These cold adaptation (ca) methods were used to introduce further attenuation in the incompletely attenuated cpRSV3131 virus in seronegative children.
[0267] In the first strategy, parental stocks of cold-passaged RSV A2 (cpRSV3131 ) (obtained from Flow Laboratories) were prepared by passaging in MRC-5 cells at 25°C as described in Example 1 . Briefly, cold-passaged virus was inoculated into MRC-5 or Vero cell culture monolayers at a multiplicity of infection ≥0.01, and infected cells were incubated for 3-14 days before the next passage. The virus was passaged more than 20 times at 20-22°C to obtain a...
Embodiment III
[0272] Introduction of additional attenuating mutations into tsRSV
[0273] This example describes the use of a ts mutant as a parental virus to generate a more fully attenuated virus strain. For this purpose, two RSV A2ts mutants, ts-4 and ts-1 NG1, were selected. Two different approaches were chosen to introduce additional mutations into RSVts mutants. The first method is: carry out chemical mutagenesis on incompletely attenuated RSVts mutant strains, and select the mutagenized progenies with more sensitive plaque formation temperature for further analysis. The second method is to passage RSV ts mutant strains at low temperature and select ts mutant strains with ca phenotype, that is, mutant strains with higher replication ability than wild-type parental virus at suboptimal temperature.
[0274] Parental stocks of the ts-1 NG1 virus were prepared from live respiratory syncytial virus (A-2) ts-1 NG-1 mutant strains from Flow Laboratories Lot M4, virus grow...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com