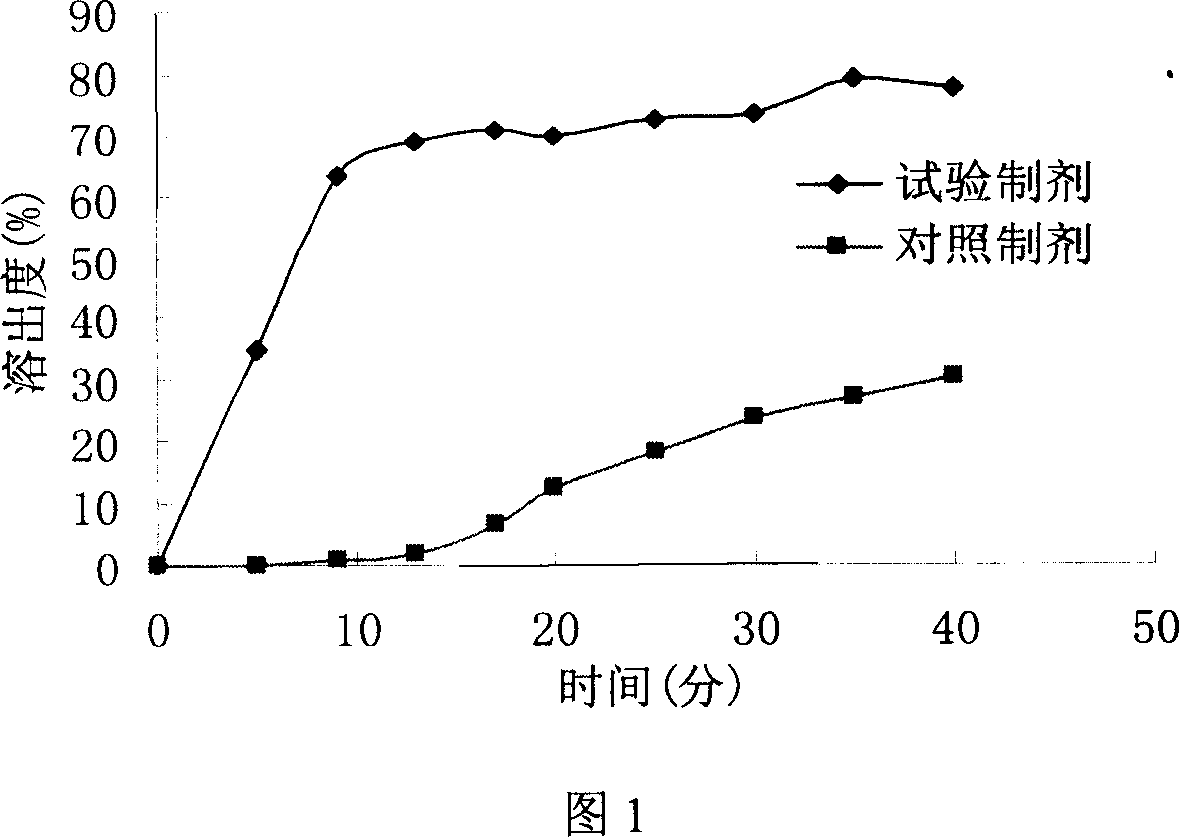
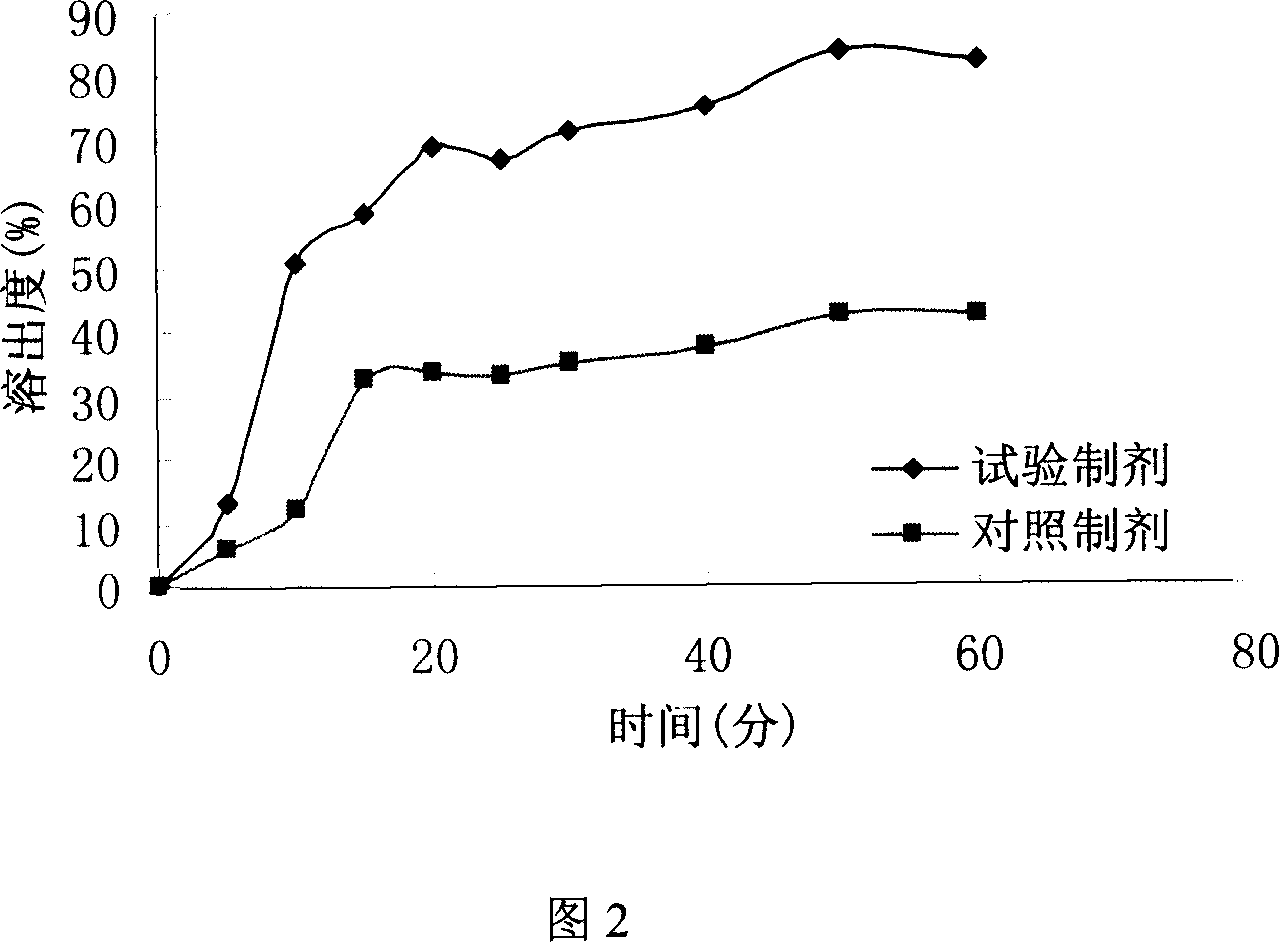
Liquid composition containing biphenyldimethy-dicarboxylate mutual soluble with water
A bifendate and solution technology, applied in the field of liquid pharmaceutical compositions, can solve the problems of reduced patient compliance, inconvenience in taking bifendate drop pills, etc., and achieves guaranteed bioavailability and is suitable for large Industrial production, the effect of simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of Bifendate Solution System
[0038] Weigh 7.5g of the bifendate raw drug, add it into a container filled with 1000ml PEG-400 solution, and stir until it is completely dissolved. Heat may be used to facilitate dissolution if necessary. Determination of content by HPLC method, that is, too.
Embodiment 2
[0040] Preparation of Bifendate Solution System
[0041] Weigh 7.5g of the bifendate raw drug, add 1.5g of Tween 80, mix well, add PEG-400 to 500ml, and stir until completely dissolved; you can also dissolve Tween 80 in the PEG-400 solution first, and then add Bifendate raw drug, stirred until completely dissolved. Heat may be used to facilitate dissolution if necessary. Determination of content by HPLC method, that is, too.
Embodiment 3
[0043] Preparation of Bifendate Solution System
[0044] Weigh 7.5 g of the bifendate raw drug, add it to a container containing 1000 ml of ethylene glycol monoethyl ether (Transcutol), and stir until it is completely dissolved. Heat may be used to facilitate dissolution if necessary. Determination of content by HPLC method, that is, too.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com