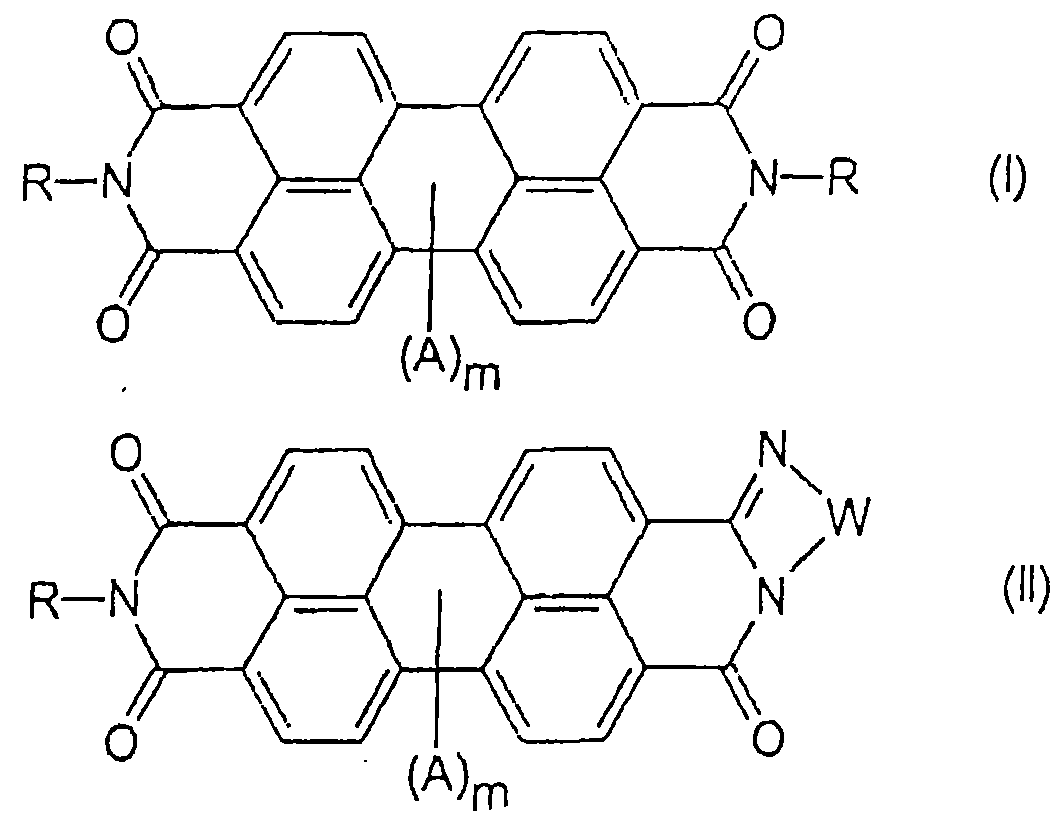
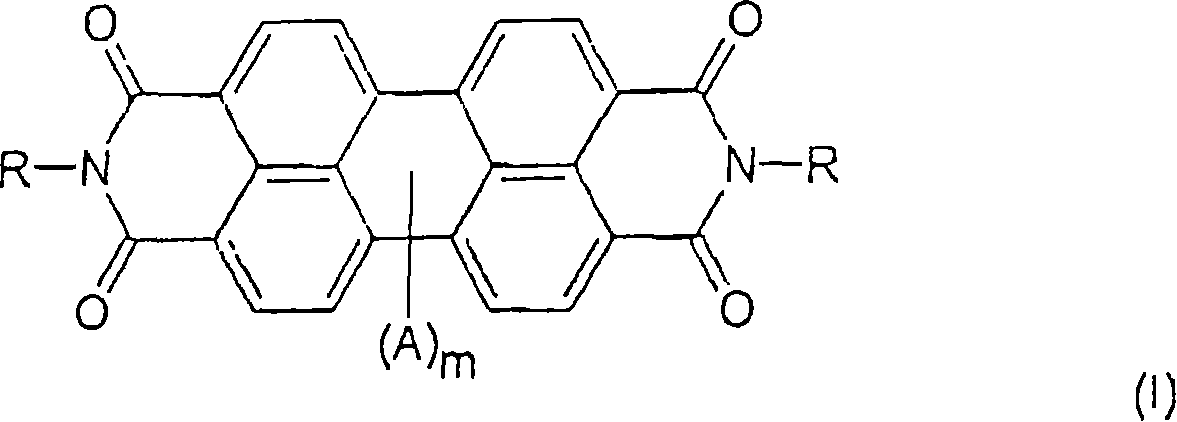
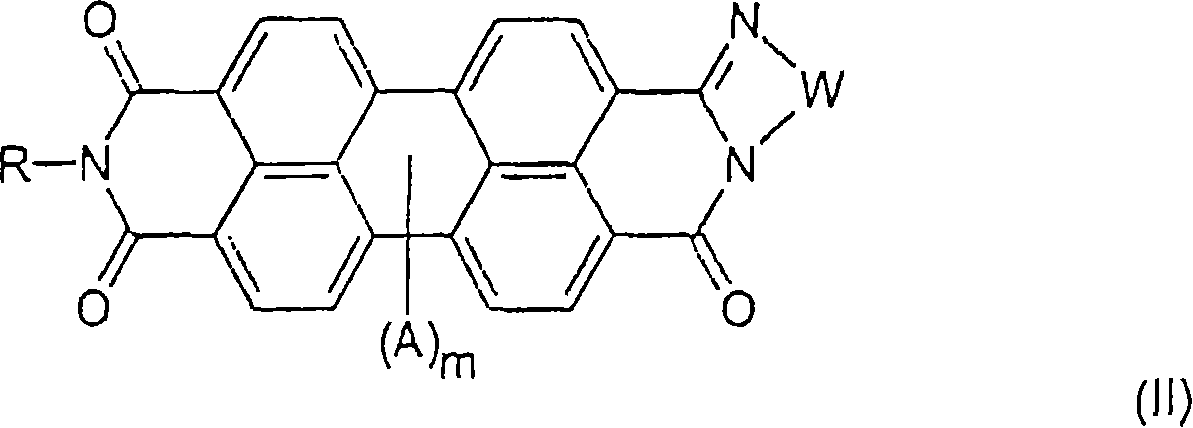
Process for making perylene pigment compositions.
A kind of perylene pigment and perylene technology are applied in the field of manufacturing perylene pigment compositions, and can solve the problems such as the preparation of perylene diimide is not described
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-5
[0075] Examples 1 and 2 describe the preparation of perylene dicarbamidine imide compositions. Comparative Example 3 describes the preparation of N,N'-dimethylperylene tetracarboxylic diimide (Pigment Red 179) without the perylene dicarbamidine imide of the present invention. Examples 4 and 5 describe the preparation of N,N'-dimethylperylene tetracarboxylic diimide in the presence of perylene dicarbamidine imide.
[0076] Particle Size Test Method
[0077] The particle size of the compositions prepared in the examples below was determined by QLS laser light scattering, DCP discs and X-ray powder diffraction. For laser light scattering and disc centrifugation, each pigment composition sample was diluted with water, dispersed with an ultrasonic signal (600W for two minutes), and further diluted. For X-ray determination, powder samples are used.
[0078] Using a Brookhaven Instruments Laser Scattering Particle Size Analyzer equipped with a BI-9000 correlator detector, a photom...
Embodiment 1
[0082] To 500 ml of deionized water in an autoclave were added 7 g of perylene monoimide / monoanhydride and 2.6 g of 2,2-dimethyl-1,3-propanediamine. The autoclave was sealed and heated and stirred at 140°C for 14 hours. The autoclave was cooled, and the contents were collected by vacuum filtration, and washed with deionized water until none flowed out. The filter cake was reslurried in 300 ml of deionized water and the pH of the suspension was adjusted to 12 with 12% aqueous potassium hydroxide. The suspension was heated at 80°C for 2 hours, cooled to 60°C and filtered. The filter cake was washed with deionized water until no flow-through occurred. A total of 20.5 g of filter cake was obtained, corresponding to 4.79 g of (VIII).
[0083]
Embodiment 2
[0085] To 500 ml of deionized water in an autoclave was added 10 g of perylene monoimide / monoanhydride and 6.91 g of 1,3-diamino-2-hydroxypropane. A solution of 4.91 g of 96% sulfur in 50 ml of deionized water was added with stirring. The autoclave was sealed and heated and stirred at 140°C for 14 hours. The autoclave was cooled, and the precipitate was collected by vacuum filtration and washed with deionized water until nothing flowed out. A filter cake of about 81 g was obtained, corresponding to about 10.1 g (IX).
[0086]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com