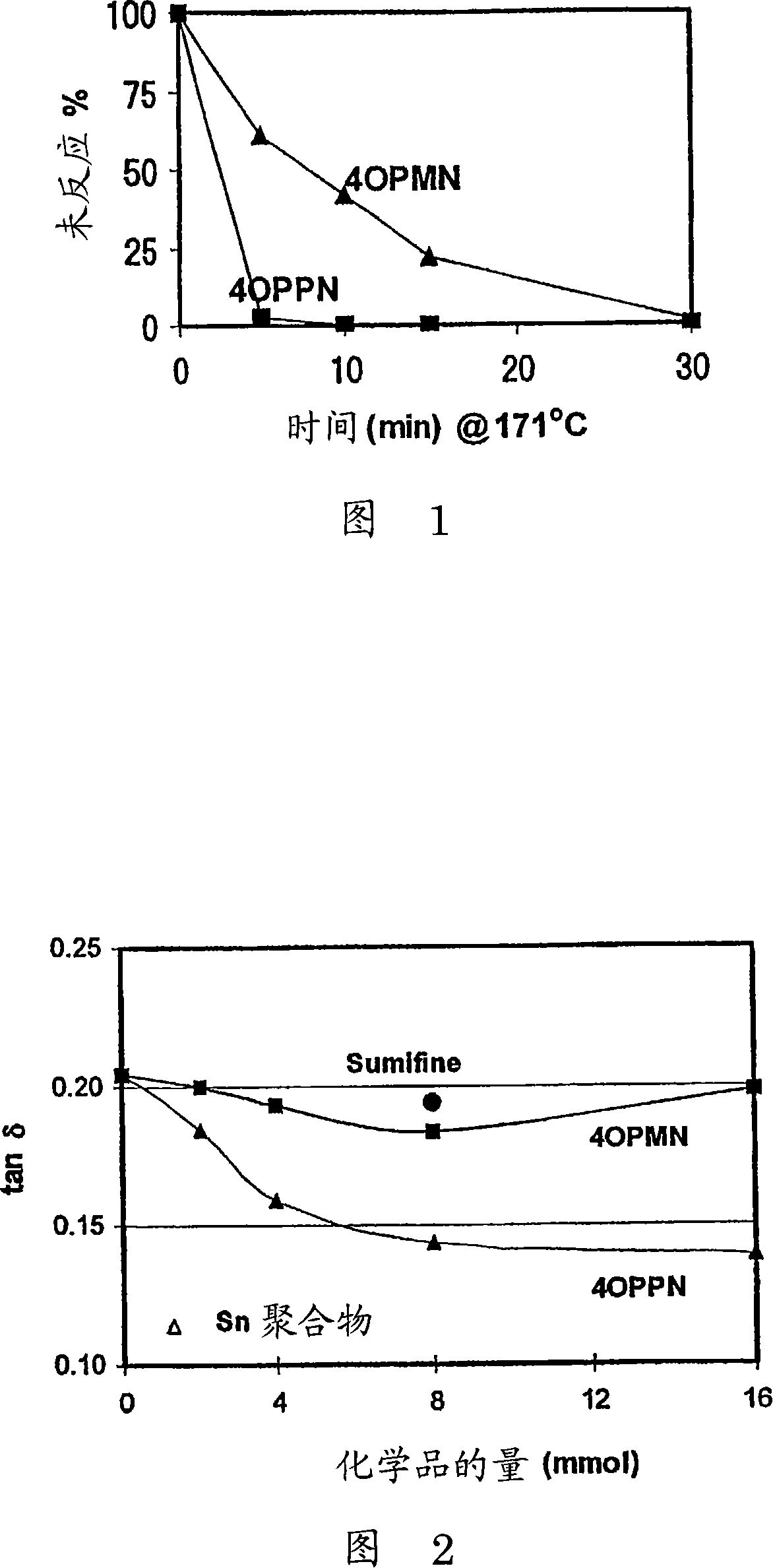
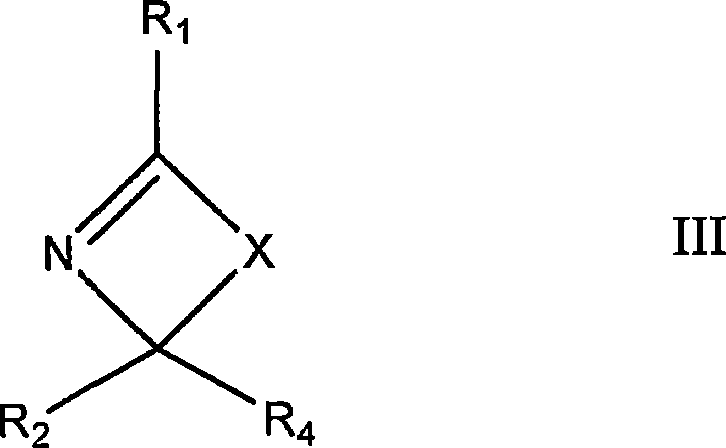
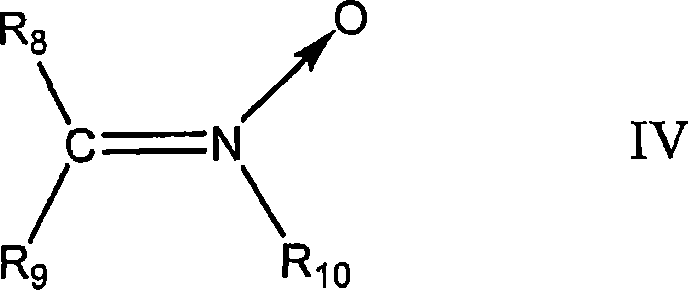
Polymer-filler coupling additives
A polymer and compound technology, applied in chemical/physical processes, transportation and packaging, organic chemistry, etc., can solve problems such as dispersion and mutual aggregation contact reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] Preparation of 4-(2-oxazolyl)-phenyl-N-phenylnitrone (4OPPN)
[0112] To a stirred mixture of 15.0 gm of 4-formyl-benzoyl chloride (1 equivalent, eq.) in 300 ml of chloroform, 2-aminoethanol (2 eq.) in chloroform (200 ml) was added dropwise at -10°C. 10.9gm solution in . After the addition, the resulting mixture was stirred at 25°C for 2 hours, and the resulting white precipitate was removed by filtration. Then, the filtrate was dried on a rotary evaporator to obtain 17.4 gm of a yellow liquid, 4-formyl-N-(2-hydroxyethyl)-benzamide.
[0113]Concentrated sulfuric acid (50 ml) was added dropwise to 4-formyl-N-(2-hydroxyethyl)-benzamide (17.4 gm) with stirring, and the mixture was heated at 100° C. for 1 hour. This solution was added dropwise to a mixture of sodium hydroxide (20%, 500ml) and chloroform (500ml) with stirring, keeping the temperature below 15°C by cooling. The organic phase is then separated off and dried. 6.3 gm of 4-(2-oxazolyl)benzaldehyde was colle...
Embodiment 2
[0116] Preparation of 4-(2-oxazolyl)-phenyl-N-methylnitrone (4OPMN)
[0117] To a stirred mixture of 4-formyl-benzoyl chloride (15.0gm, 89mmol) in chloroform (300ml) was added dropwise 2-aminoethanol (10.9gm, 178mmol) in chloroform (200ml) at -10°C. ) solution. After the addition, the resulting mixture was stirred at 25°C for 2 hours, then the white precipitate was filtered. The filtrate was dried by rotary evaporation to obtain 17 gm (88 mmol) of yellow liquid, 4-formyl-N-(2-hydroxyethyl)-benzamide (99% yield).
[0118] Concentrated sulfuric acid (50ml) was added dropwise to 4-formyl-N-(2-hydroxyethyl)-benzamide (17gm, 88mmol) with stirring, and the mixture was heated at 100°C for 1 hour. This solution was added dropwise to a mixture of sodium hydroxide (20%, 500ml) and chloroform (500ml) with stirring, keeping the temperature below 15°C by cooling. The organic phase is then separated off and dried. 6.3 gm (36 mMol) of 4-(2-oxazolyl)benzaldehyde was collected (41% yield...
Embodiment 3
[0121] Preparation of Phenyl-N-[4-(2-oxazolyl)phenyl]nitrone (P4OPN)
[0122] A solution of p-nitro-benzoyl chloride (185.6 mg, 100 mol) in 450 mL of hot benzene was added to a mixture of ethanolamine (63.5 g, 1.04 mol) in 1350 mL of water. Then 830 ml of 5% sodium hydroxide solution were gradually added. A white precipitate comprising p-nitro-N-(2-hydroxyethyl)benzamide formed which was filtered and dried. The dried p-nitro-N-(2-hydroxyethyl)benzamide powder weighed 196 g (0.93 mol) (93% yield).
[0123] Thionyl chloride (132 mL, 1.8 mol) was added dropwise to 196 g of p-nitro-N-(2-hydroxyethyl)benzamide powder with stirring. After a vigorous reaction, the resulting mixture was poured into 1 L of diethyl ether. An insoluble material comprising p-nitro-N-(2-chloroethyl)benzamide formed in ether which was filtered and dried to leave a white powder. 192 g (0.84 mol) of p-nitro-N-(2-chloroethyl)benzamide are obtained (90% yield).
[0124] 192 g of p-nitro-N-(2-chloroethyl)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com