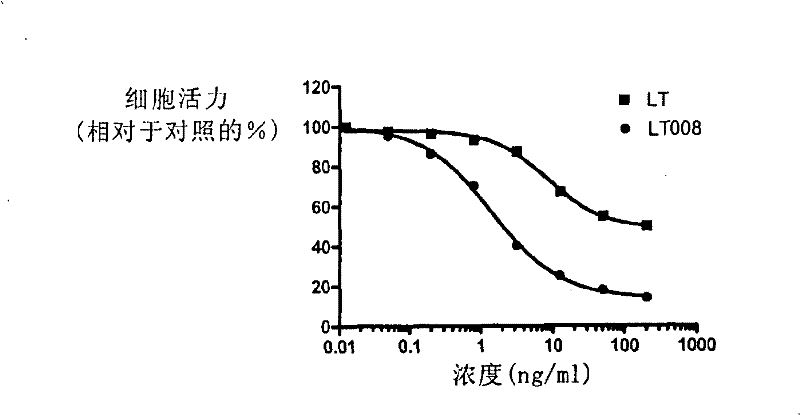
Receptor selectivity lymphotoxin derivates
A lymphotoxin, selective technology for protein engineering and pharmaceutical applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Example 1 Wild-type LT, LT 24-171 , LT 28-171 preparation of
[0084] Expression vector construction:
[0085] According to the human LT sequence (BAA00064) in GENBANK, Shanghai Sangon Biotechnology Service Co., Ltd. was commissioned to synthesize the wild-type LT encoding the 1st to 171st, 24th to 171th, and 28th to 171th positions of the LT mature protein. , LT 24-171 , LT 28-171 Amino acid sequence, and cloned on the NdeI and HindIII sites of the pET32a (+) vector (purchased from Novagen), to obtain recombinant expression plasmids LT / pET32a (+), LT 24-171 / pET32a(+), LT 28-171 / pET32a(+).
[0086] Expression in E. coli:
[0087] Recombinant expression plasmid LT / pET32a(+), LT 24-171 / pET32a(+), LT 28-171 / pET32a(+) were transformed into Escherichia coli BL21(DE3), and the transformation liquid was coated on LB (containing ampicillin 100ng / μl) plate. Pick a single clone from the transformation plate and inoculate it in 1 mL of LB medium. After culturing at 3...
Embodiment 2
[0091] The preparation of embodiment 2 mutant rhLT008
[0092] Construction of rhLT008 mutant expression vector:
[0093] (1) PCR amplification mutant gene:
[0094] to LT 24-171 / pET32a(+) plasmid was used as a template, and LT-P32U and Q107E-R were used as a pair of primers to amplify to obtain the LT008-AB fragment. CD fragments. Then, LT008-AB and LT008-CD were mixed as a template, and LT-P32U and LT-P32D were used as a pair of primers to amplify to obtain the LT008 fragment containing the Q107E mutation.
[0095] LT-P32U: 5' ACACATATGATG CAC TCT ACC CTG AAA CCG 3' (SEQ ID NO: 4)
[0096] LT-P32D: 5' TGCAAGCTTCTA CAG AGC GAA GGC TCC AAA 3' (SEQ ID NO: 5)
[0097] Q107E-F: 5' CTCTTCTCCTCCGAATACCCCTTC 3' (SEQ ID NO: 6)
[0098] Q107E-R: 5'GAAGGGGTATTCGGAGGAGAAGAG 3' (SEQ ID NO: 7)
[0099] After purification, the PCR product was ligated with the pMD-18T vector, transformed into Escherichia coli DH5a, extracted the LT008 / pMD-18T plasmid, and sequenced it in forward and...
Embodiment 3
[0105] Embodiment 3 Preparation of LT006
[0106] to LT 24-171 / pET32a(+) plasmid was used as a template, and LT-P32U and S106E-Q107E-R were used as a pair of primers to amplify to obtain the LT006-AB fragment, and at the same time, LT-P32D and S106E-Q107E-F were used to amplify , to obtain the LT006-CD fragment. Then LT006-AB and LT006-CD were mixed as a template, and LT-P32U and LT-P32D were used as a pair of primers to amplify to obtain the full-length LT006 fragment containing the S106E-Q107E mutation.
[0107] The PCR primer sequences are:
[0108] S106E-Q107E-F: 5' CTCTTCTCCGAAGAATACCCCCTTC 3' (SEQ ID NO: 8)
[0109] S106E-Q107E-R: 5'GAAGGGGTATTCTTCGGAGAAGAG 3' (SEQ ID NO: 9)
[0110] Cloning, expression, and purification of LT006 were carried out in the same manner as in Example 2 to obtain 3.1 mg of LT006 protein.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com