Novel substituted thiophenepyrimidinone derivatives as inhibitors of 17beta-hydroxysteroid dehydrogenase
A hydroxyl and substituent technology, applied in the field of thienyl pyrimidone derivatives, can solve the problems of membrane permeability difficulties and insolubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0382] In order to more fully illustrate the nature of the invention and the mode of carrying it out, the following examples are provided but should not be construed as limiting.
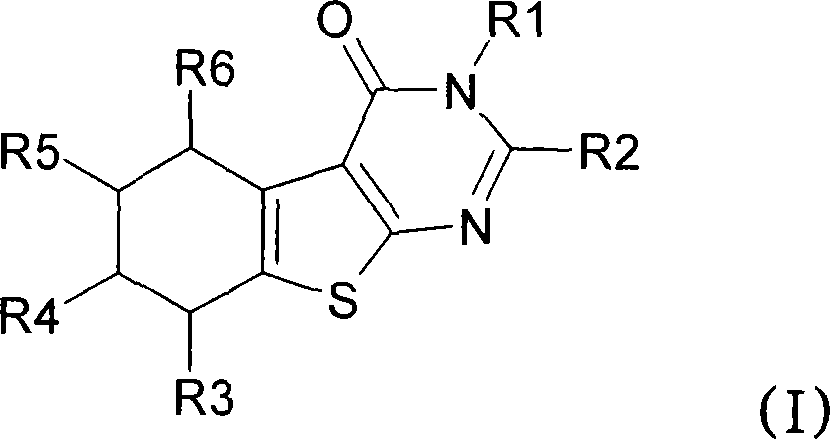
[0383] Preparation of the following No.1 to No.17 compounds falling within the scope of general formula (I):
[0384] Compound No.1: 3-Benzyl-5-methyl-2-(3,4,5-trimethoxyphenyl)-5,6,7,8-tetrahydro-3H-benzo[4,5 ]thieno[2,3-d]pyrimidin-4-one
[0385]
[0386] 2-Amino-4-methyl-4,5,6,7-tetrahydro-benzo[b]-thiophene-3-carboxylic acid ethyl ester (26.6mmol, 100mol-%) and N-benzyl-3, 4,5-Trimethoxy-benzamide (34.6 mmol, 130 mol-%) was dissolved in anhydrous 1,2-dichloroethane. Cool the reaction mixture with an ice-salt bath, add POCl 3 (1.7ml, 24.6mmol, 130mol-%). The reaction mixture was refluxed for 24 hours. Add POCl twice during reflux 3 (340 μl). The reaction mixture was poured into ice water, and after neutralization with sodium acetate, the product was extracted into dichloromethane. The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com