Stable supersaturated gemcitabine hydrochloride solution and its prepn process
A gemcitabine hydrochloride solution technology, which is applied in the field of supersaturated drug solution and its preparation, can solve the problems of difficult preparation of high-concentration injections and increase of degradation products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
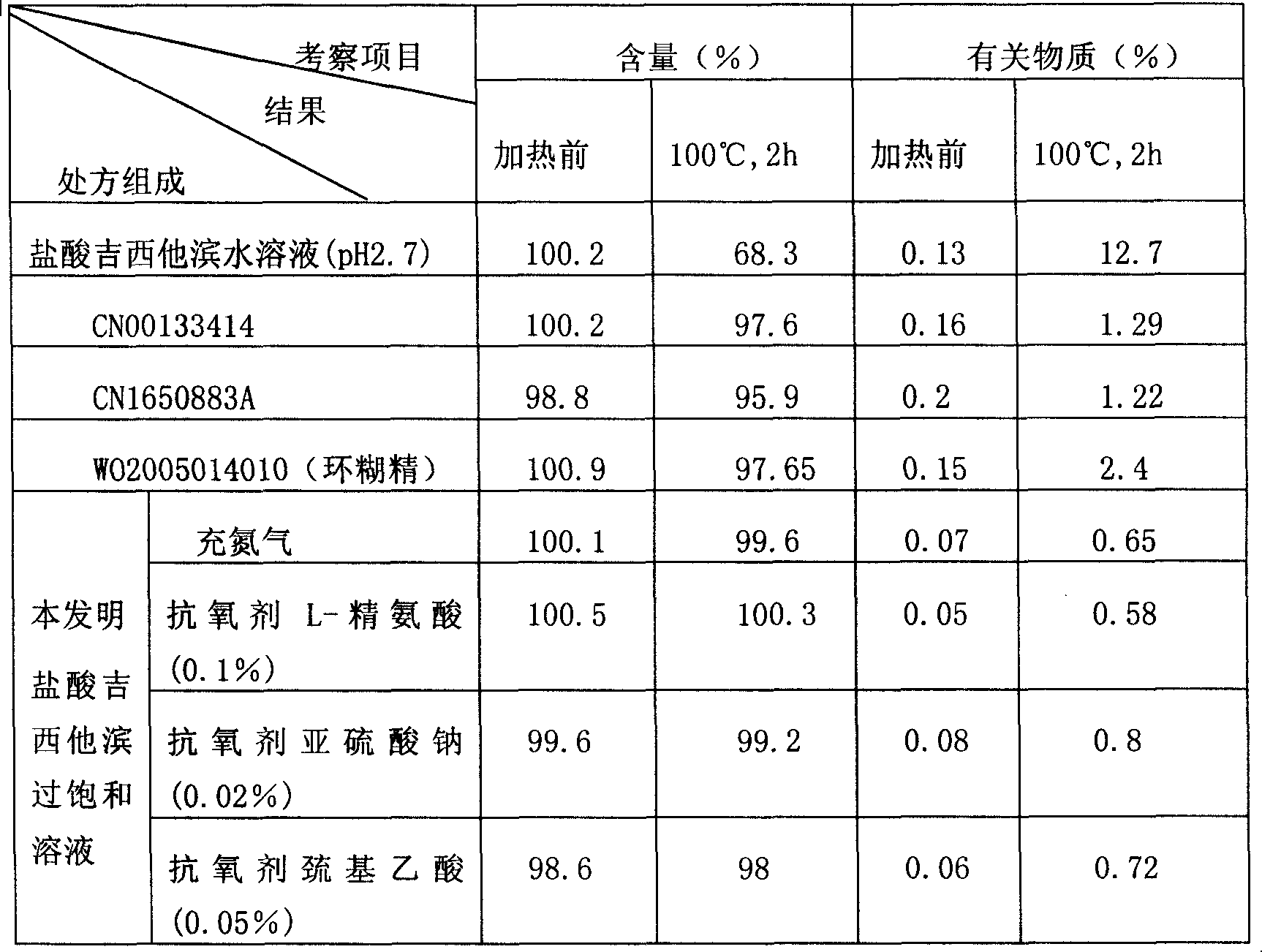
[0034] Example 1: Get 227.7 mg of gemcitabine hydrochloride (containing 200 mg of gemcitabine), dissolve it with 6 ml of water for injection, add 0.09 g of sodium chloride, and then add 0.5 N sodium hydroxide to adjust the pH to 4.5. The above solution is heated to 30-40 ° C, Dissolve the precipitated crystals completely, and dilute to 10ml with water for injection. Then sterilize and filter while it is hot, pack in 10ml ampoules, each 10ml, and seal.
Embodiment 2
[0035] Embodiment 2: get gemcitabine hydrochloride 341.6mg (containing gemcitabine 300mg), dissolve with 6ml water for injection, add 0.09g sodium chloride, then add 0.5N sodium hydroxide, adjust pH to be 6.0, above-mentioned solution is heated to 50~60 ℃, Dissolve the precipitated crystals completely, and dilute to 10ml with water for injection. Then sterilize and filter while hot, pack in ampoules, fill with nitrogen, and seal.
Embodiment 3
[0036]Embodiment 3: get gemcitabine hydrochloride 455mg (containing gemcitabine 400mg), dissolve with 6ml water for injection, add 0.09g sodium chloride, then add 0.5N sodium hydroxide, adjust pH to be 5.5, above-mentioned solution is heated to 60~80 ℃, makes The precipitated crystals were completely dissolved and diluted to 10ml with water for injection. Then sterilize and filter while hot, pack in 5ml ampoules, each 5ml, fill with nitrogen, and seal.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com